Bad Actors Getting Your Health Data Is the FBI’s Latest Worry

A hacker activating a 3D rendering of DNA data.
In February 2015, the health insurer Anthem revealed that criminal hackers had gained access to the company's servers, exposing the personal information of nearly 79 million patients. It's the largest known healthcare breach in history.
FBI agents worry that the vast amounts of healthcare data being generated for precision medicine efforts could leave the U.S. vulnerable to cyber and biological attacks.
That year, the data of millions more would be compromised in one cyberattack after another on American insurers and other healthcare organizations. In fact, for the past several years, the number of reported data breaches has increased each year, from 199 in 2010 to 344 in 2017, according to a September 2018 analysis in the Journal of the American Medical Association.
The FBI's Edward You sees this as a worrying trend. He says hackers aren't just interested in your social security or credit card number. They're increasingly interested in stealing your medical information. Hackers can currently use this information to make fake identities, file fraudulent insurance claims, and order and sell expensive drugs and medical equipment. But beyond that, a new kind of cybersecurity threat is around the corner.
Mr. You and others worry that the vast amounts of healthcare data being generated for precision medicine efforts could leave the U.S. vulnerable to cyber and biological attacks. In the wrong hands, this data could be used to exploit or extort an individual, discriminate against certain groups of people, make targeted bioweapons, or give another country an economic advantage.
Precision medicine, of course, is the idea that medical treatments can be tailored to individuals based on their genetics, environment, lifestyle or other traits. But to do that requires collecting and analyzing huge quantities of health data from diverse populations. One research effort, called All of Us, launched by the U.S. National Institutes of Health last year, aims to collect genomic and other healthcare data from one million participants with the goal of advancing personalized medical care.
Other initiatives are underway by academic institutions and healthcare organizations. Electronic medical records, genetic tests, wearable health trackers, mobile apps, and social media are all sources of valuable healthcare data that a bad actor could potentially use to learn more about an individual or group of people.
"When you aggregate all of that data together, that becomes a very powerful profile of who you are," Mr. You says.
A supervisory special agent in the biological countermeasures unit within the FBI's weapons of mass destruction directorate, it's Mr. You's job to imagine worst-case bioterror scenarios and figure out how to prevent and prepare for them.
That used to mean focusing on threats like anthrax, Ebola, and smallpox—pathogens that could be used to intentionally infect people—"basically the dangerous bugs," as he puts it. In recent years, advances in gene editing and synthetic biology have given rise to fears that rogue, or even well-intentioned, scientists could create a virulent virus that's intentionally, or unintentionally, released outside the lab.
"If a foreign source, especially a criminal one, has your biological information, then they might have some particular insights into what your future medical needs might be and exploit that."
While Mr. You is still tracking those threats, he's been traveling around the country talking to scientists, lawyers, software engineers, cyber security professionals, government officials and CEOs about new security threats—those posed by genetic and other biological data.
Emerging threats
Mr. You says one possible situation he can imagine is the potential for nefarious actors to use an individual's sensitive medical information to extort or blackmail that person.
"If a foreign source, especially a criminal one, has your biological information, then they might have some particular insights into what your future medical needs might be and exploit that," he says. For instance, "what happens if you have a singular medical condition and an outside entity says they have a treatment for your condition?" You could get talked into paying a huge sum of money for a treatment that ends up being bogus.
Or what if hackers got a hold of a politician or high-profile CEO's health records? Say that person had a disease-causing genetic mutation that could affect their ability to carry out their job in the future and hackers threatened to expose that information. These scenarios may seem far-fetched, but Mr. You thinks they're becoming increasingly plausible.
On a wider scale, Kavita Berger, a scientist at Gryphon Scientific, a Washington, D.C.-area life sciences consulting firm, worries that data from different populations could be used to discriminate against certain groups of people, like minorities and immigrants.
For instance, the advocacy group Human Rights Watch in 2017 flagged a concerning trend in China's Xinjiang territory, a region with a history of government repression. Police there had purchased 12 DNA sequencers and were collecting and cataloging DNA samples from people to build a national database.
"The concern is that this particular province has a huge population of the Muslim minority in China," Ms. Berger says. "Now they have a really huge database of genetic sequences. You have to ask, why does a police station need 12 next-generation sequencers?"
Also alarming is the potential that large amounts of data from different groups of people could lead to customized bioweapons if that data ends up in the wrong hands.
Eleonore Pauwels, a research fellow on emerging cybertechnologies at United Nations University's Centre for Policy Research, says new insights gained from genomic and other data will give scientists a better understanding of how diseases occur and why certain people are more susceptible to certain diseases.
"As you get more and more knowledge about the genomic picture and how the microbiome and the immune system of different populations function, you could get a much deeper understanding about how you could target different populations for treatment but also how you could eventually target them with different forms of bioagents," Ms. Pauwels says.
Economic competitiveness
Another reason hackers might want to gain access to large genomic and other healthcare datasets is to give their country a leg up economically. Many large cyber-attacks on U.S. healthcare organizations have been tied to Chinese hacking groups.
"This is a biological space race and we just haven't woken up to the fact that we're in this race."
"It's becoming clear that China is increasingly interested in getting access to massive data sets that come from different countries," Ms. Pauwels says.
A year after U.S. President Barack Obama conceived of the Precision Medicine Initiative in 2015—later renamed All of Us—China followed suit, announcing the launch of a 15-year, $9 billion precision health effort aimed at turning China into a global leader in genomics.
Chinese genomics companies, too, are expanding their reach outside of Asia. One company, WuXi NextCODE, which has offices in Shanghai, Reykjavik, and Cambridge, Massachusetts, has built an extensive library of genomes from the U.S., China and Iceland, and is now setting its sights on Ireland.
Another Chinese company, BGI, has partnered with Children's Hospital of Philadelphia and Sinai Health System in Toronto, and also formed a collaboration with the Smithsonian Institute to sequence all species on the planet. BGI has built its own advanced genomic sequencing machines to compete with U.S.-based Illumina.
Mr. You says having access to all this data could lead to major breakthroughs in healthcare, such as new blockbuster drugs. "Whoever has the largest, most diverse dataset is truly going to win the day and come up with something very profitable," he says.
Some direct-to-consumer genetic testing companies with offices in the U.S., like Dante Labs, also use BGI to process customers' DNA.
Experts worry that China could race ahead the U.S. in precision medicine because of Chinese laws governing data sharing. Currently, China prohibits the exportation of genetic data without explicit permission from the government. Mr. You says this creates an asymmetry in data sharing between the U.S. and China.
"This is a biological space race and we just haven't woken up to the fact that we're in this race," he said in January at an American Society for Microbiology conference in Washington, D.C. "We don't have access to their data. There is absolutely no reciprocity."
Protecting your data
While Mr. You has been stressing the importance of data security to anyone who will listen, the National Academies of Sciences, Engineering, and Medicine, which makes scientific and policy recommendations on issues of national importance, has commissioned a study on "safeguarding the bioeconomy."
In the meantime, Ms. Berger says organizations that deal with people's health data should assess their security risks and identify potential vulnerabilities in their systems.
As for what individuals can do to protect themselves, she urges people to think about the different ways they're sharing healthcare data—such as via mobile health apps and wearables.
"Ask yourself, what's the benefit of sharing this? What are the potential consequences of sharing this?" she says.
Mr. You also cautions people to think twice before taking consumer DNA tests. They may seem harmless, he says, but at the end of the day, most people don't know where their genetic information is going. "If your genetic sequence is taken, once it's gone, it's gone. There's nothing you can do about it."
60-Second Video: How Safe Are the COVID-19 Vaccines?
Comparing the Risks of COVID-19 vs. the Risks of the Vaccines:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
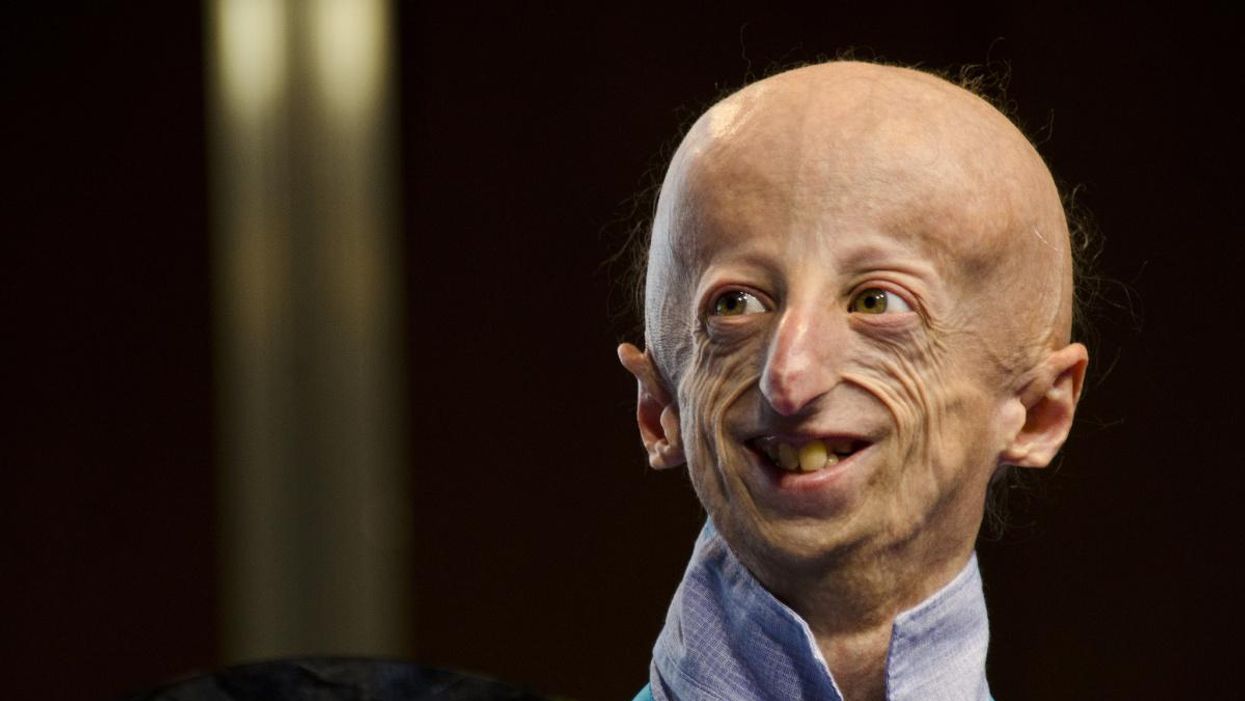
For Kids with Progeria, New Therapies May Offer Revolutionary Hope for a Longer Life
Sammy Basso is one of around 400 young people in the world with progeria.
Sammy Basso has some profound ideas about fate. As long as he has been alive, he has known he has minimal control over his own. His parents, however, had to transition from a world of unlimited possibility to one in which their son might not live to his 20s.
"I remember very clearly that day because Sammy was three years old," his mother says of the day a genetic counselor diagnosed Sammy with progeria. "It was a devastating day for me."
But to Sammy, he has always been himself: a smart kid, interested in science, a little smaller than his classmates, with one notable kink in his DNA. In one copy of the gene that codes for the protein Lamin A, Sammy has a T where there should be a C. The incorrect code creates a toxic protein called progerin, which destabilizes Sammy's cells and makes him age much faster than a person who doesn't have the mutation. The older he gets, the more he is in danger of strokes, heart failure, or a heart attack. "I am okay with my situation," he says from his home in Tezze sul Brenta, Italy. "But I think, yes, fate has a great role in my life."
Just 400 or so people in the world live with progeria: The mutation that causes it usually arises de novo, or "of new," meaning that it is not inherited but happens spontaneously during gestation. The challenge, as with all rare diseases, is that few cases means few treatments.
"When we first started, there was absolutely nothing out there," says Leslie Gordon, a physician-researcher who co-founded the Progeria Research Foundation in 1999 after her own son, also named Sam, was diagnosed with the disease. "We knew we had to jumpstart the entire field, so we collected money through road races and special events and writing grants and all sorts of donors… I think the first year we raised $75,000, most of it from one donor."
"We have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body."
By 2003, the foundation had collaborated with Francis Collins, a geneticist who is now director of the National Institutes of Health, to work out the genetic basis for progeria—that single mutation Sammy has. The discovery led to interest in lonafarnib, a drug that was already being used in cancer patients but could potentially operate downstream of the mutation, preventing the buildup of the defective progerin in the body. "We funded cellular studies to look at a lonafarnib in cells, mouse studies to look at lonafarnib in mouse models of progeria… and then we initiated the clinical trials," Gordon says.
Sammy Basso's family had gotten involved with the Progeria Research Foundation through their international patient registry, which maintains relationships with families in 49 countries. "We started to hear about lonafarnib in 2006 from Leslie Gordon," says Sammy's father, Amerigo Basso, with his son translating. "She told us about the lonafarnib. And we were very happy because for the first time we understood that there was something that could help our son and our lives." Amerigo used the Italian word speranza, which means hope.
Still, Sammy wasn't sure if lonafarnib was right for him. "Since when I was very young I thought that everything happens for a reason. So, in my mind, if God made me with progeria, there was a reason, and to try to heal from progeria was something wrong," he says. Gradually, his parents and doctors, and Leslie Gordon, convinced him otherwise. Sammy began to believe that God was also the force behind doctors, science, and research. "And so we have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body," he says.

Sammy Basso and his parents.
Courtesy of Basso
Sammy began taking lonafarnib, with the Progeria Research Foundation intermittently flying him, and other international trial participants, to Boston for tests. He was immediately beset by some of the drug's more unpleasant side effects: Stomach problems, nausea, and vomiting. "The first period was absolutely the worst period of my life," he says.
At first, doctors prescribed other medicines for the side effects, but to Sammy it had as much effect as drinking water. He visited doctor after doctor, with some calling him weekly or even daily to ask how he was doing. Eventually the specialists decided that he should lower his dose, balancing his pain with the benefit of the drug. Sammy can't actually feel any positive effect of the lonafarnib, but his health measurements have improved relative to people with progeria who don't take it.
While they never completely disappeared, Sammy's side effects decreased to the point that he could live. Inspired by the research that led to lonafarnib, he went to university to study molecular biology. For his thesis work, he travelled to Spain to perform experiments on cells and on mice with progeria, learning how to use the gene-editing technique CRISPR-Cas9 to cut out the mutated bit of DNA. "I was so excited to participate in this study," Sammy says. He felt like his work could make a difference.
In 2018, the Progeria Research Foundation was hosting one of their biennial workshops when Francis Collins, the researcher who had located the mutation behind progeria 15 years earlier, got in touch with Leslie Gordon. "Francis called me and said, Hey, I just saw a talk by David Liu from the Broad [Institute]. And it was pretty amazing. He has been looking at progeria and has very early, but very exciting data… Do you have any spaces, any slots you could make in your program for late breaking news?"
Gordon found a spot, and David Liu came to talk about what was going on in his lab, which was an even more advanced treatment that led to mice with the progeria mutation living into their senior mouse years—substantially closer to a normal lifespan. Liu's lab had built on the idea of CRISPR-Cas9 to create a more elegant genetic process called base editing: Instead of chopping out mutated DNA, a scientist could chemically convert an incorrect DNA letter to the correct one, like the search and replace function in word processing software. Mice who had their Lamin-A mutations corrected this way lived more than twice as long as untreated animals.
Sammy was in the audience at Dr. Liu's talk. "When I heard about this base editing as a younger scientist, I thought that I was living in the future," he says. "When my parents had my diagnosis of progeria, the science knew very little information about DNA. And now we are talking about healing the DNA… It is incredible."
Lonafarnib (also called Zokinvy) was approved by the US Food and Drug Administration this past November. Sammy, now 25, still takes it, and still manages his side effects. With luck, the gift of a few extra years will act as a bridge until he can try Liu's revolutionary new gene treatment, which has not yet begun testing in humans. While Leslie Gordon warns that she's always wrong about things like this, she hopes to see the new base editing techniques in clinical trials in the next year or two. Sammy won't need to be convinced to try it this time; his thinking on fate has evolved since his first encounter with lonafarnib.
"I would be very happy to try it," he says. "I know that for a non-scientist it can be difficult to understand. Some people think that we are the DNA. We are not. The DNA is a part of us, and to correct it is to do what we are already doing—just better." In short, a gene therapy, while it may seem like science fiction, is no different from a pill. For Sammy, both are a new way to think about fate: No longer something that simply happens to him.