Beyond Henrietta Lacks: How the Law Has Denied Every American Ownership Rights to Their Own Cells

A 2017 portrait of Henrietta Lacks.
The common perception is that Henrietta Lacks was a victim of poverty and racism when in 1951 doctors took samples of her cervical cancer without her knowledge or permission and turned them into the world's first immortalized cell line, which they called HeLa. The cell line became a workhorse of biomedical research and facilitated the creation of medical treatments and cures worth untold billions of dollars. Neither Lacks nor her family ever received a penny of those riches.
But racism and poverty is not to blame for Lacks' exploitation—the reality is even worse. In fact all patients, then and now, regardless of social or economic status, have absolutely no right to cells that are taken from their bodies. Some have called this biological slavery.
How We Got Here
The case that established this legal precedent is Moore v. Regents of the University of California.
John Moore was diagnosed with hairy-cell leukemia in 1976 and his spleen was removed as part of standard treatment at the UCLA Medical Center. On initial examination his physician, David W. Golde, had discovered some unusual qualities to Moore's cells and made plans prior to the surgery to have the tissue saved for research rather than discarded as waste. That research began almost immediately.
"On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery.'"
Even after Moore moved to Seattle, Golde kept bringing him back to Los Angeles to collect additional samples of blood and tissue, saying it was part of his treatment. When Moore asked if the work could be done in Seattle, he was told no. Golde's charade even went so far as claiming to find a low-income subsidy to pay for Moore's flights and put him up in a ritzy hotel to get him to return to Los Angeles, while paying for those out of his own pocket.
Moore became suspicious when he was asked to sign new consent forms giving up all rights to his biological samples and he hired an attorney to look into the matter. It turned out that Golde had been lying to his patient all along; he had been collecting samples unnecessary to Moore's treatment and had turned them into a cell line that he and UCLA had patented and already collected millions of dollars in compensation. The market for the cell lines was estimated at $3 billion by 1990.
Moore felt he had been taken advantage of and filed suit to claim a share of the money that had been made off of his body. "On both sides of the case, legal experts and cultural observers cautioned that ownership of a human body was the first step on the slippery slope to 'bioslavery,'" wrote Priscilla Wald, a professor at Duke University whose career has focused on issues of medicine and culture. "Moore could be viewed as asking to commodify his own body part or be seen as the victim of the theft of his most private and inalienable information."
The case bounced around different levels of the court system with conflicting verdicts for nearly six years until the California Supreme Court ruled on July 9, 1990 that Moore had no legal rights to cells and tissue once they were removed from his body.
The court made a utilitarian argument that the cells had no value until scientists manipulated them in the lab. And it would be too burdensome for researchers to track individual donations and subsequent cell lines to assure that they had been ethically gathered and used. It would impinge on the free sharing of materials between scientists, slow research, and harm the public good that arose from such research.
"In effect, what Moore is asking us to do is impose a tort duty on scientists to investigate the consensual pedigree of each human cell sample used in research," the majority wrote. In other words, researchers don't need to ask any questions about the materials they are using.
One member of the court did not see it that way. In his dissent, Stanley Mosk raised the specter of slavery that "arises wherever scientists or industrialists claim, as defendants have here, the right to appropriate and exploit a patient's tissue for their sole economic benefit—the right, in other words, to freely mine or harvest valuable physical properties of the patient's body. … This is particularly true when, as here, the parties are not in equal bargaining positions."
Mosk also cited the appeals court decision that the majority overturned: "If this science has become for profit, then we fail to see any justification for excluding the patient from participation in those profits."
But the majority bought the arguments that Golde, UCLA, and the nascent biotechnology industry in California had made in amici briefs filed throughout the legal proceedings. The road was now cleared for them to develop products worth billions without having to worry about or share with the persons who provided the raw materials upon which their research was based.
Critical Views
Biomedical research requires a continuous and ever-growing supply of human materials for the foundation of its ongoing work. If an increasing number of patients come to feel as John Moore did, that the system is ripping them off, then they become much less likely to consent to use of their materials in future research.
Some legal and ethical scholars say that donors should be able to limit the types of research allowed for their tissues and researchers should be monitored to assure compliance with those agreements. For example, today it is commonplace for companies to certify that their clothing is not made by child labor, their coffee is grown under fair trade conditions, that food labeled kosher is properly handled. Should we ask any less of our pharmaceuticals than that the donors whose cells made such products possible have been treated honestly and fairly, and share in the financial bounty that comes from such drugs?
Protection of individual rights is a hallmark of the American legal system, says Lisa Ikemoto, a law professor at the University of California Davis. "Putting the needs of a generalized public over the interests of a few often rests on devaluation of the humanity of the few," she writes in a reimagined version of the Moore decision that upholds Moore's property claims to his excised cells. The commentary is in a chapter of a forthcoming book in the Feminist Judgment series, where authors may only use legal precedent in effect at the time of the original decision.
"Why is the law willing to confer property rights upon some while denying the same rights to others?" asks Radhika Rao, a professor at the University of California, Hastings College of the Law. "The researchers who invest intellectual capital and the companies and universities that invest financial capital are permitted to reap profits from human research, so why not those who provide the human capital in the form of their own bodies?" It might be seen as a kind of sweat equity where cash strapped patients make a valuable in kind contribution to the enterprise.
The Moore court also made a big deal about inhibiting the free exchange of samples between scientists. That has become much less the situation over the more than three decades since the decision was handed down. Ironically, this decision, as well as other laws and regulations, have since strengthened the power of patents in biomedicine and by doing so have increased secrecy and limited sharing.
"Although the research community theoretically endorses the sharing of research, in reality sharing is commonly compromised by the aggressive pursuit and defense of patents and by the use of licensing fees that hinder collaboration and development," Robert D. Truog, Harvard Medical School ethicist and colleagues wrote in 2012 in the journal Science. "We believe that measures are required to ensure that patients not bear all of the altruistic burden of promoting medical research."
Additionally, the increased complexity of research and the need for exacting standardization of materials has given rise to an industry that supplies certified chemical reagents, cell lines, and whole animals bred to have specific genetic traits to meet research needs. This has been more efficient for research and has helped to ensure that results from one lab can be reproduced in another.
The Court's rationale of fostering collaboration and free exchange of materials between researchers also has been undercut by the changing structure of that research. Big pharma has shrunk the size of its own research labs and over the last decade has worked out cooperative agreements with major research universities where the companies contribute to the research budget and in return have first dibs on any findings (and sometimes a share of patent rights) that come out of those university labs. It has had a chilling effect on the exchange of materials between universities.
Perhaps tracking cell line donors and use restrictions on those donations might have been burdensome to researchers when Moore was being litigated. Some labs probably still kept their cell line records on 3x5 index cards, computers were primarily expensive room-size behemoths with limited capacity, the internet barely existed, and there was no cloud storage.
But that was the dawn of a new technological age and standards have changed. Now cell lines are kept in state-of-the-art sub zero storage units, tagged with the source, type of tissue, date gathered and often other information. Adding a few more data fields and contacting the donor if and when appropriate does not seem likely to disrupt the research process, as the court asserted.
Forging the Future
"U.S. universities are awarded almost 3,000 patents each year. They earn more than $2 billion each year from patent royalties. Sharing a modest portion of these profits is a novel method for creating a greater sense of fairness in research relationships that we think is worth exploring," wrote Mark Yarborough, a bioethicist at the University of California Davis Medical School, and colleagues. That was penned nearly a decade ago and those numbers have only grown.
The Michigan BioTrust for Health might serve as a useful model in tackling some of these issues. Dried blood spots have been collected from all newborns for half a century to be tested for certain genetic diseases, but controversy arose when the huge archive of dried spots was used for other research projects. As a result, the state created a nonprofit organization to in essence become a biobank and manage access to these spots only for specific purposes, and also to share any revenue that might arise from that research.
"If there can be no property in a whole living person, does it stand to reason that there can be no property in any part of a living person? If there were, can it be said that this could equate to some sort of 'biological slavery'?" Irish ethicist Asim A. Sheikh wrote several years ago. "Any amount of effort spent pondering the issue of 'ownership' in human biological materials with existing law leaves more questions than answers."
Perhaps the biggest question will arise when -- not if but when -- it becomes possible to clone a human being. Would a human clone be a legal person or the property of those who created it? Current legal precedent points to it being the latter.
Today, October 4, is the 70th anniversary of Henrietta Lacks' death from cancer. Over those decades her immortalized cells have helped make possible miraculous advances in medicine and have had a role in generating billions of dollars in profits. Surviving family members have spoken many times about seeking a share of those profits in the name of social justice; they intend to file lawsuits today. Such cases will succeed or fail on their own merits. But regardless of their specific outcomes, one can hope that they spark a larger public discussion of the role of patients in the biomedical research enterprise and lead to establishing a legal and financial claim for their contributions toward the next generation of biomedical research.
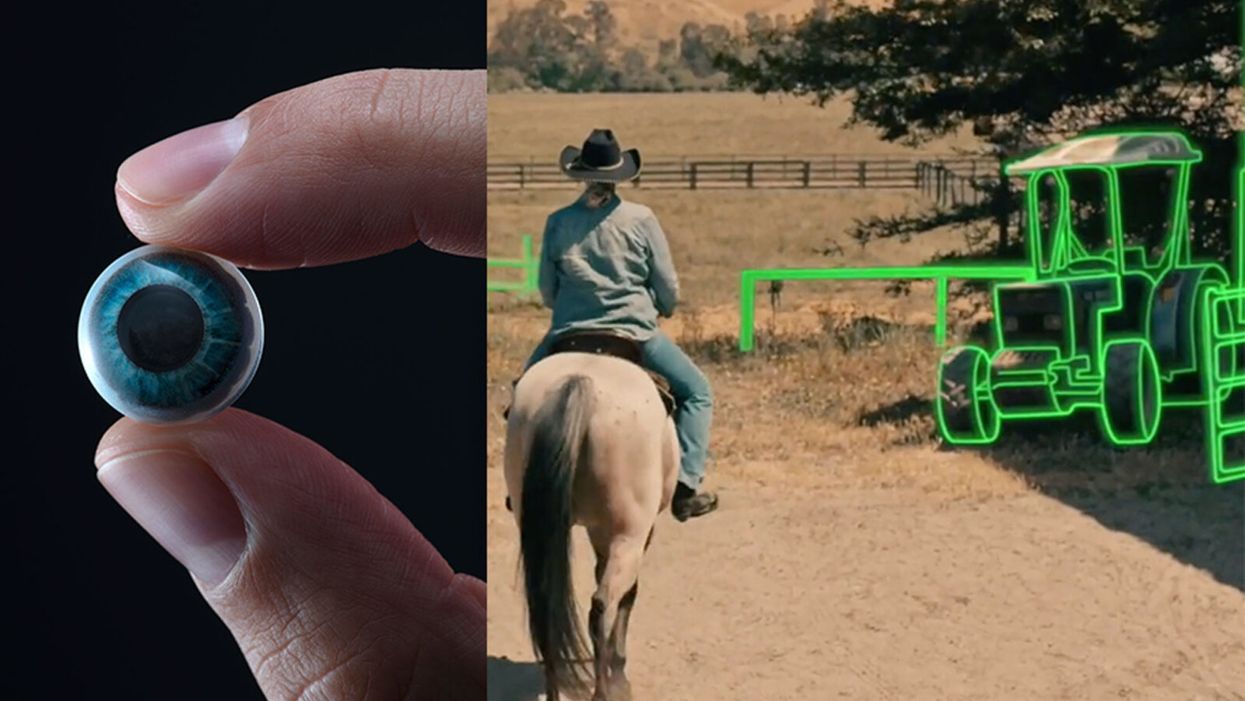
World’s First “Augmented Reality” Contact Lens Aims to Revolutionize Much More Than Medicine
On the left, a picture of the Mojo lens smart contact; and a simulated image of a woman with low vision who is wearing the contact to highlight objects in her field of vision.
Imagine a world without screens. Instead of endlessly staring at your computer or craning your neck down to scroll through social media feeds and emails, information simply appears in front of your eyes when you need it and disappears when you don't.
"The vision is super clear...I was reading the poem with my eyes closed."
No more rude interruptions during dinner, no more bumping into people on the street while trying to follow GPS directions — just the information you want, when you need it, projected directly onto your visual field.
While this screenless future sounds like science fiction, it may soon be a reality thanks to the new Silicon Valley startup Mojo Vision, creator of the world's first smart contact lens. With a 14,000 pixel-per-inch display with eye-tracking, image stabilization, and a custom wireless radio, the Mojo smart lens bills itself the "smallest and densest dynamic display ever made." Unlike current augmented reality wearables such as Google Glass or ThirdEye, which project images onto a glass screen, the Mojo smart lens can project images directly onto the retina.
A current prototype displayed at the Consumer Electronics Show earlier this year in Las Vegas includes a tiny screen positioned right above the most sensitive area of the pupil. "[The Mojo lens] is a contact lens that essentially has wireless power and data transmission for a small micro LED projector that is placed over the center of the eye," explains David Hobbs, Director of Product Management at Mojo Vision. "[It] displays critical heads-up information when you need it and fades into the background when you're ready to continue on with your day."
Eventually, Mojo Visions' technology could replace our beloved smart devices but the first generation of the Mojo smart lens will be used to help the 2.2 billion people globally who suffer from vision impairment.
"If you think of the eye as a camera [for the visually impaired], the sensors are not working properly," explains Dr. Ashley Tuan, Vice President of Medical Devices at Mojo Vision and fellow of the American Academy of Optometry. "For this population, our lens can process the image so the contrast can be enhanced, we can make the image larger, magnify it so that low-vision people can see it or we can make it smaller so they can check their environment." In January of this year, the FDA granted Breakthrough Device Designation to Mojo, allowing them to have early and frequent discussions with the FDA about technical, safety and efficacy topics before clinical trials can be done and certification granted.
For now, Dr. Tuan is one of the few people who has actually worn the Mojo lens. "I put the contact lens on my eye. It was very comfortable like any contact lenses I've worn before," she describes. "The vision is super clear and then when I put on the accessories, suddenly I see Yoda in front of me and I see my vital signs. And then I have my colleague that prepared a beautiful poem that I loved when I was young [and] I was reading the poem with my eyes closed."
At the moment, there are several electronic glasses on the market like Acesight and Nueyes Pro that provide similar solutions for those suffering from visual impairment, but they are large, cumbersome, and highly visible. Mojo lens would be a discreet, more comfortable alternative that offers users more freedom of movement and independence.
"In the case of augmented-reality contact lenses, there could be an opportunity to improve the lives of people with low vision," says Dr. Thomas Steinemann, spokesperson for the American Academy of Ophthalmology and professor of ophthalmology at MetroHealth Medical Center in Cleveland. "There are existing tools for people currently living with low vision—such as digital apps, magnifiers, etc.— but something wearable could provide more flexibility and significantly more aid in day-to-day tasks."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless.
According to Dr. Tuan, the visually impaired often suffer from depression due to their lack of mobility and 70 percent of them are underemployed. "We hope that they can use this device to gain their mobility so they can get that social aspect back in their lives and then, eventually, employment," she explains. "That is our first and most important goal."
But helping those with low visual capabilities is only Mojo lens' first possible medical application; augmented reality is already being used in medicine and is poised to revolutionize the field in the coming decades. For example, Accuvein, a device that uses lasers to provide real-time images of veins, is widely used by nurses and doctors to help with the insertion of needles for IVs and blood tests.
According to the National Center for Biotechnology Information, augmentation of reality has been used in surgery for many years with surgeons using devices such as Google Glass to overlay critical information about their patients into their visual field. Using software like the Holographic Navigation Platform by Scopsis, surgeons can see a mixed-reality overlay that can "show you complicated tumor boundaries, assist with implant placements and guide you along anatomical pathways," its developers say.
However, according to Dr. Tuan, augmented reality headsets have drawbacks in the surgical setting. "The advantage of [Mojo lens] is you don't need to worry about sweating or that the headset or glasses will slide down to your nose," she explains "Also, our lens is designed so that it will understand your intent, so when you don't want the image overlay it will disappear, it will not block your visual field, and when you need it, it will come back at the right time."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless. Possibilities include live translation of sign language for deaf people; helping those with autism to read emotions; and improving doctors' bedside manner by allowing them to fully engage with patients without relying on a computer.
"[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
Furthermore, the lens could be used to monitor health issues. "We have image sensors in the lens right now that point to the world but we can have a camera pointing inside of your eye to your retina," says Dr. Tuan, "[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
For the moment, the future medical applications of the Mojo lens are still theoretical, but the team is confident they can eventually become a reality after going through the proper regulatory review. The company is still in the process of design, prototype and testing of the lens, so they don't know exactly when it will be available for use, but they anticipate shipping the first available products in the next couple of years. Once it does go to market, it will be available by prescription only for those with visual impairments, but the team's goal is to bring it to broader consumer markets pending regulatory clearance.
"We see that right now there's a unique opportunity here for Mojo lens and invisible computing to help to shape what the next decade of technology development looks like," explains David Hobbs. "We can use [the Mojo lens] to better serve us as opposed to us serving technology better."
Schmidt Ocean Institute co-founder Wendy Schmidt is backed by 32 screens in research vessel Falkor's control room where most of the science takes place on the ship, from mapping to live streaming of underwater robotic dives.
WENDY SCHMIDT is a philanthropist and investor who has spent more than a dozen years creating innovative non-profit organizations to solve pressing global environmental and human rights issues. Recognizing the human dependence on sustaining and protecting our planet and its people, Wendy has built organizations that work to educate and advance an understanding of the critical interconnectivity between the land and the sea. Through a combination of grants and investments, Wendy's philanthropic work supports research and science, community organizations, promising leaders, and the development of innovative technologies. Wendy is president of The Schmidt Family Foundation, which she co-founded with her husband Eric in 2006. They also co-founded Schmidt Ocean Institute and Schmidt Futures.
Editors: The pandemic has altered the course of human history and the nature of our daily lives in equal measure. How has it affected the focus of your philanthropy across your organizations? Have any aspects of the crisis in particular been especially galvanizing as you considered where to concentrate your efforts?
Wendy: The COVID-19 pandemic has made the work of our philanthropy more relevant than ever. If anything, the circumstances of this time have validated the focus we have had for nearly 15 years. We support the need for universal access to clean, renewable energy, healthy food systems, and the dignity of human labor and self-determination in a world of interconnected living systems on land and in the Ocean we are only beginning to understand.
When you consider the disproportionate impact of the COVID-19 virus on people who are poorly paid, poorly housed, with poor nutrition and health care, and exposed to unsafe conditions in the workplace—you see clearly how the systems that have been defining how we live, what we eat, who gets healthcare and what impacts the environment around us—need to change.
"This moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science."
If the pandemic teaches us anything, we learn what resilience looks like, and the essential role for local small businesses including restaurants, farms and ranches, dairies and fish markets in the long term vitality of communities. There is resonance, local economic benefit, and also accountability in these smaller systems, with shorter supply chains and less vertical integration.
The consolidation of vertically integrated business operations for the sake of global efficiency reveals its essential weakness when supply chains break down and the failure to encourage local economic centers leads to intense systemic disruption and the possibility of collapse.
Editors: For scientists, one significant challenge has been figuring out how to continue research, if at all, during this time of isolation and distancing. Yet, your research vessel Falkor, of the Schmidt Ocean Institute, is still on its expedition exploring the Coral Sea Marine Park in Australia—except now there are no scientists onboard. What was the vessel up to before the pandemic hit? Can you tell us more about how they are continuing to conduct research from afar now and how that's going?
Wendy: We have been extremely fortunate at Schmidt Ocean Institute. When the pandemic hit in March, our research vessel, Falkor, was already months into a year-long program to research unexplored deep sea canyons around Australia and at the Great Barrier Reef. We were at sea, with an Australian science group aboard, carrying on with our mission of exploration, discovery and communication, when we happened upon what we believe to be the world's longest animal—a siphonophore about 150 feet long, spiraling out at a depth of about 2100 feet at the end of a deeper dive in the Ningaloo Canyon off Western Australia. It was the kind of wondrous creature we find so often when we conduct ROV dives in the world's Ocean.
For more than two months this year, Falkor was reportedly the only research vessel in the world carrying on active research at sea. Once we were able to dock and return the science party to shore, we resumed our program at sea offering a scheduled set of now land-based scientists in lockdown in Australia the opportunity to conduct research remotely, taking advantage of the vessel's ship to shore communications, high resolution cameras and live streaming video. It's a whole new world, and quite wonderful in its own way.
Editors: Normally, 10–15 scientists would be aboard such a vessel. Is "remote research" via advanced video technology here to stay? Are there any upsides to this "new normal"?
Wendy: Like all things pandemic, remote research is an adaptation for what would normally occur. Since we are putting safety of the crew and guest scientists at the forefront, we're working to build strong remote connections between our crew, land based scientists and the many robotic tools on board Falkor. There's no substitute for in person work, but what we've developed during the current cruise is a pretty good and productive alternative in a crisis. And what's important is that this critical scientific research into the deep sea is able to continue, despite the pandemic on land.
Editors: Speaking of marine expeditions, you've sponsored two XPRIZE competitions focused on ocean health. Do you think challenge prizes could fill gaps of the global COVID-19 response, for example, to manufacture more testing kits, accelerate the delivery of PPE, or incentivize other areas of need?
Wendy: One challenge we are currently facing is that innovations don't have the funding pathway to scale, so promising ideas by entrepreneurs, researchers, and even major companies are being developed too slowly. Challenge prizes help raise awareness for problems we are trying to solve and attract new people to help solve those problems by giving them a pathway to contribute.
One idea might be for philanthropy to pair prizes and challenges with an "advanced market commitment" where the government commits to a purchase order for the innovation if it meets a certain test. That could be deeply impactful for areas like PPE and the production of testing kits.
Editors: COVID-19 testing, especially, has been sorely needed, here in the U.S. and in developing countries as well as low-income communities. That's why we're so intrigued by your Schmidt Science Fellows grantee Hal Holmes and his work to repurpose a new DNA technology to create a portable, mobile test for COVID-19. Can you tell us about that work and how you are supporting it?
Wendy: Our work with Conservation X Labs began years ago when our foundation was the first to support their efforts to develop a handheld DNA barcode sensor to help detect illegally imported and mislabeled seafood and timber products. The device was developed by Hal Holmes, who became one of our Schmidt Science Fellows and is the technical lead on the project, working closely with Conservation X Labs co-founders Alex Deghan and Paul Bunje. Now, with COVID-19, Hal and team have worked with another Schmidt Science Fellow, Fahim Farzardfard, to repurpose the technology—which requires no continuous power source, special training, or a lab—to serve as a mobile testing device for the virus.
The work is going very well, manufacturing is being organized, and distribution agreements with hospitals and government agencies are underway. You could see this device in use within a few months and have testing results within hours instead of days. It could be especially useful in low-income communities and developing countries where access to testing is challenging.
Editors: How is Schmidt Futures involved in the development of information platforms that will offer productive solutions?
Wendy: In addition to the work I've mentioned, we've also funded the development of tech-enabled tools that can help the medical community be better prepared for the ongoing spike of COVID cases. For example, we funded EdX and Learning Agency to develop an online training to help increase the number of medical professionals who can operate ventilators. The first course is being offered by Harvard University, and so far, over 220,000 medical professionals have enrolled. We have also invested in informational platforms that make it easier to contain the spread of the disease, such as our work with Recidiviz to model the impact of COVID-19 in prisons and outline policy steps states could take to limit the spread.
Information platforms can also play a big part pushing forward scientific research into the virus. For example, we've funded the UC Santa Cruz Virus Browser, which allows researchers to examine each piece of the virus and see the proteins it creates, the interactions in the host cell, and — most importantly — almost everything the recent scientific literature has to say about that stretch of the molecule.
Editors: The scale of research collaboration and the speed of innovation today seem unprecedented. The whole science world has turned its attention to combating the pandemic. What positive big-picture trends do you think or hope will persist once the crisis eventually abates?
Wendy: As in many areas, the COVID crisis has accelerated trends in the scientific world that were already well underway. For instance, this moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science.
We believe collaboration is an essential ingredient for progress in all areas. Early in this pandemic, Schmidt Futures held a virtual gathering of 160 people across 70 organizations in philanthropy, government, and business interested in accelerating research and response to the virus, and thought at the time, it's pretty amazing this kind of thing doesn't go all the time. We are obviously going to go farther together than on our own...
My husband, Eric, has observed that in the past two months, we've all catapulted 10 years forward in our use of technology, so there are trends already underway that are likely accelerated and will become part of the fabric of the post-COVID world—like working remotely; online learning; increased online shopping, even for groceries; telemedicine; increasing use of AI to create smarter delivery systems for healthcare and many other applications in a world that has grown more virtual overnight.
"Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part."
We fully expect these trends to continue and expand across the sciences, sped up by the pressures of the health crisis. Schmidt Ocean Institute and Schmidt Futures have been pressing in these directions for years, so we are pleased to see the expansions that should help more scientists work productively, together.
Editors: Trying to find the good amid a horrible crisis, are there any other new horizons in science, philanthropy, and/or your own work that could transform our world for the better that you'd like to share?
Wendy: Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part. The more we investigate the Ocean, the more we look deeply into what lies in our soils and beneath them, the more we realize we do not know, and moreover, how vulnerable humanity is to the forces of the natural world.
Philanthropy has an important role to play in influencing how people perceive our place in the world and understand the impact of human activity on the rest of the planet. I believe it's philanthropy's role to take risks, to invest early in innovative technologies, to lead where governments and industry aren't ready to go yet. We're fortunate at this time to be able to help those working on tools to better diagnose and treat the virus, and to invest in those working to improve information systems, so citizens and policy makers can make better decisions that can reduce impacts on families and institutions.
From all we know, this isn't likely to be the last pandemic the world will see. It's been said that a crisis comes before change, and we would hope that we can play a role in furthering the work to build systems that are resilient—in information, energy, agriculture and in all the ways we work, recreate, and use the precious resources of our planet.
[This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.