Biologists are Growing Mini-Brains. What If They Become Conscious?

Brain coral metaphorically represents the concept of growing organoids in a dish that may eventually attain consciousness--if scientists can first determine what that means.
Few images are more uncanny than that of a brain without a body, fully sentient but afloat in sterile isolation. Such specters have spooked the speculatively-minded since the seventeenth century, when René Descartes declared, "I think, therefore I am."
Since August 29, 2019, the prospect of a bodiless but functional brain has begun to seem far less fantastical.
In Meditations on First Philosophy (1641), the French penseur spins a chilling thought experiment: he imagines "having no hands or eyes, or flesh, or blood or senses," but being tricked by a demon into believing he has all these things, and a world to go with them. A disembodied brain itself becomes a demon in the classic young-adult novel A Wrinkle in Time (1962), using mind control to subjugate a planet called Camazotz. In the sci-fi blockbuster The Matrix (1999), most of humanity endures something like Descartes' nightmare—kept in womblike pods by their computer overlords, who fill the captives' brains with a synthetized reality while tapping their metabolic energy as a power source.
Since August 29, 2019, however, the prospect of a bodiless but functional brain has begun to seem far less fantastical. On that date, researchers at the University of California, San Diego published a study in the journal Cell Stem Cell, reporting the detection of brainwaves in cerebral organoids—pea-size "mini-brains" grown in the lab. Such organoids had emitted random electrical impulses in the past, but not these complex, synchronized oscillations. "There are some of my colleagues who say, 'No, these things will never be conscious,'" lead researcher Alysson Muotri, a Brazilian-born biologist, told The New York Times. "Now I'm not so sure."

Alysson Muotri has no qualms about his creations attaining consciousness as a side effect of advancing medical breakthroughs.
(Credit: ZELMAN STUDIOS)
Muotri's findings—and his avowed ambition to push them further—brought new urgency to simmering concerns over the implications of brain organoid research. "The closer we come to his goal," said Christof Koch, chief scientist and president of the Allen Brain Institute in Seattle, "the more likely we will get a brain that is capable of sentience and feeling pain, agony, and distress." At the annual meeting of the Society for Neuroscience, researchers from the Green Neuroscience Laboratory in San Diego called for a partial moratorium, warning that the field was "perilously close to crossing this ethical Rubicon and may have already done so."
Yet experts are far from a consensus on whether brain organoids can become conscious, whether that development would necessarily be dreadful—or even how to tell if it has occurred.
So how worried do we need to be?
***
An organoid is a miniaturized, simplified version of an organ, cultured from various types of stem cells. Scientists first learned to make them in the 1980s, and have since turned out mini-hearts, lungs, kidneys, intestines, thyroids, and retinas, among other wonders. These creations can be used for everything from observation of basic biological processes to testing the effects of gene variants, pathogens, or medications. They enable researchers to run experiments that might be less accurate using animal models and unethical or impractical using actual humans. And because organoids are three-dimensional, they can yield insights into structural, developmental, and other matters that an ordinary cell culture could never provide.
In 2006, Japanese biologist Shinya Yamanaka developed a mix of proteins that turned skin cells into "pluripotent" stem cells, which could subsequently be transformed into neurons, muscle cells, or blood cells. (He later won a Nobel Prize for his efforts.) Developmental biologist Madeline Lancaster, then a post-doctoral student at the Institute of Molecular Biotechnology in Vienna, adapted that technique to grow the first brain organoids in 2013. Other researchers soon followed suit, cultivating specialized mini-brains to study disorders ranging from microcephaly to schizophrenia.
Muotri, now a youthful 45-year-old, was among the boldest of these pioneers. His team revealed the process by which Zika virus causes brain damage, and showed that sofosbuvir, a drug previously approved for hepatitis C, protected organoids from infection. He persuaded NASA to fly his organoids to the International Space Station, where they're being used to trace the impact of microgravity on neurodevelopment. He grew brain organoids using cells implanted with Neanderthal genes, and found that their wiring differed from organoids with modern DNA.
Like the latter experiment, Muotri's brainwave breakthrough emerged from a longtime obsession with neuroarchaeology. "I wanted to figure out how the human brain became unique," he told me in a phone interview. "Compared to other species, we are very social. So I looked for conditions where the social brain doesn't function well, and that led me to autism." He began investigating how gene variants associated with severe forms of the disorder affected neural networks in brain organoids.
Tinkering with chemical cocktails, Muotri and his colleagues were able to keep their organoids alive far longer than earlier versions, and to culture more diverse types of brain cells. One team member, Priscilla Negraes, devised a way to measure the mini-brains' electrical activity, by planting them in a tray lined with electrodes. By four months, the researchers found to their astonishment, normal organoids (but not those with an autism gene) emitted bursts of synchronized firing, separated by 20-second silences. At nine months, the organoids were producing up to 300,000 spikes per minute, across a range of frequencies.
He shared his vision for "brain farms," which would grow organoids en masse for drug development or tissue transplants.
When the team used an artificial intelligence system to compare these patterns with EEGs of gestating fetuses, the program found them to be nearly identical at each stage of development. As many scientists noted when the news broke, that didn't mean the organoids were conscious. (Their chaotic bursts bore little resemblance to the orderly rhythms of waking adult brains.) But to some observers, it suggested that they might be approaching the borderline.
***
Shortly after Muotri's team published their findings, I attended a conference at UCSD on the ethical questions they raised. The scientist, in jeans and a sky-blue shirt, spoke rhapsodically of brain organoids' potential to solve scientific mysteries and lead to new medical treatments. He showed video of a spider-like robot connected to an organoid through a computer interface. The machine responded to different brainwave patterns by walking or stopping—the first stage, Muotri hoped, in teaching organoids to communicate with the outside world. He described his plans to develop organoids with multiple brain regions, and to hook them up to retinal organoids so they could "see." He shared his vision for "brain farms," which would grow organoids en masse for drug development or tissue transplants.

Muotri holds a spider-like robot that can connect to an organoid through a computer interface.
(Credit: ROLAND LIZARONDO/KPBS)
Yet Muotri also stressed the current limitations of the technology. His organoids contain approximately 2 million neurons, compared to about 200 million in a rat's brain and 86 billion in an adult human's. They consist only of a cerebral cortex, and lack many of a real brain's cell types. Because researchers haven't yet found a way to give organoids blood vessels, moreover, nutrients can't penetrate their inner recesses—a severe constraint on their growth.
Another panelist strongly downplayed the imminence of any Rubicon. Patricia Churchland, an eminent philosopher of neuroscience, cited research suggesting that in mammals, networked connections between the cortex and the thalamus are a minimum requirement for consciousness. "It may be a blessing that you don't have the enabling conditions," she said, "because then you don't have the ethical issues."
Christof Koch, for his part, sounded much less apprehensive than the Times had made him seem. He noted that science lacks a definition of consciousness, beyond an organism's sense of its own existence—"the fact that it feels like something to be you or me." As to the competing notions of how the phenomenon arises, he explained, he prefers one known as Integrated Information Theory, developed by neuroscientist Giulio Tononi. IIT considers consciousness to be a quality intrinsic to systems that reach a certain level of complexity, integration, and causal power (the ability for present actions to determine future states). By that standard, Koch doubted that brain organoids had stepped over the threshold.
One way to tell, he said, might be to use the "zap and zip" test invented by Tononi and his colleague Marcello Massimini in the early 2000s to determine whether patients are conscious in the medical sense. This technique zaps the brain with a pulse of magnetic energy, using a coil held to the scalp. As loops of neural impulses cascade through the cerebral circuitry, an EEG records the firing patterns. In a waking brain, the feedback is highly complex—neither totally predictable nor totally random. In other states, such as sleep, coma, or anesthesia, the rhythms are simpler. Applying an algorithm commonly used for computer "zip" files, the researchers devised a scale that allowed them to correctly diagnose most patients who were minimally conscious or in a vegetative state.
If scientists could find a way to apply "zap and zip" to brain organoids, Koch ventured, it should be possible to rank their degree of awareness on a similar scale. And if it turned out that an organoid was conscious, he added, our ethical calculations should strive to minimize suffering, and avoid it where possible—just as we now do, or ought to, with animal subjects. (Muotri, I later learned, was already contemplating sensors that would signal when organoids were likely in distress.)
During the question-and-answer period, an audience member pressed Churchland about how her views might change if the "enabling conditions" for consciousness in brain organoids were to arise. "My feeling is, we'll answer that when we get there," she said. "That's an unsatisfying answer, but it's because I don't know. Maybe they're totally happy hanging out in a dish! Maybe that's the way to be."
***
Muotri himself admits to no qualms about his creations attaining consciousness, whether sooner or later. "I think we should try to replicate the model as close as possible to the human brain," he told me after the conference. "And if that involves having a human consciousness, we should go in that direction." Still, he said, if strong evidence of sentience does arise, "we should pause and discuss among ourselves what to do."
"The field is moving so rapidly, you blink your eyes and another advance has occurred."
Churchland figures it will be at least a decade before anyone reaches the crossroads. "That's partly because the thalamus has a very complex architecture," she said. It might be possible to mimic that architecture in the lab, she added, "but I tend to think it's not going to be a piece of cake."
If anything worries Churchland about brain organoids, in fact, it's that Muotri's visionary claims for their potential could set off a backlash among those who find them unacceptably spooky. "Alysson has done brilliant work, and he's wonderfully charismatic and charming," she said. "But then there's that guy back there who doesn't think it's exciting; he thinks you're the Devil incarnate. You're playing into the hands of people who are going to shut you down."
Koch, however, is more willing to indulge Muotri's dreams. "Ten years ago," he said, "nobody would have believed you can take a stem cell and get an entire retina out of it. It's absolutely frigging amazing. So who am I to say the same thing can't be true for the thalamus or the cortex? The field is moving so rapidly, you blink your eyes and another advance has occurred."
The point, he went on, is not to build a Cartesian thought experiment—or a Matrix-style dystopia—but to vanquish some of humankind's most terrifying foes. "You know, my dad passed away of Parkinson's. I had a twin daughter; she passed away of sudden death syndrome. One of my best friends killed herself; she was schizophrenic. We want to eliminate all these terrible things, and that requires experimentation. We just have to go into it with open eyes."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
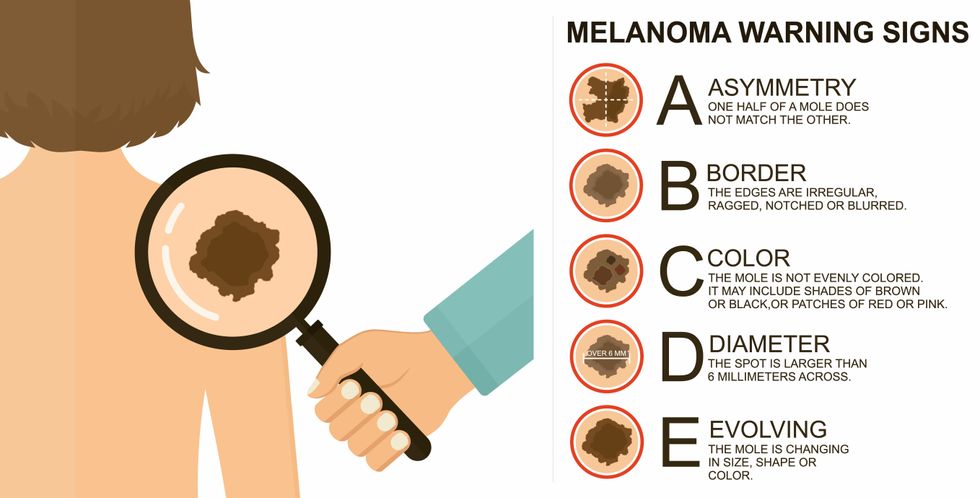
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

