Bivalent Boosters for Young Children Are Elusive. The Search Is On for Ways to Improve Access.

Theo, an 18-month-old in rural Nebraska, walks with his father in their backyard. For many toddlers, the barriers to accessing COVID-19 vaccines are many, such as few locations giving vaccines to very young children.
It’s Theo’s* first time in the snow. Wide-eyed, he totters outside holding his father’s hand. Sarah Holmes feels great joy in watching her 18-month-old son experience the world, “His genuine wonder and excitement gives me so much hope.”
In the summer of 2021, two months after Theo was born, Holmes, a behavioral health provider in Nebraska lost her grandparents to COVID-19. Both were vaccinated and thought they could unmask without any risk. “My grandfather was a veteran, and really trusted the government and faith leaders saying that COVID-19 wasn’t a threat anymore,” she says.” The state of emergency in Louisiana had ended and that was the message from the people they respected. “That is what killed them.”
The current official public health messaging is that regardless of what variant is circulating, the best way to be protected is to get vaccinated. These warnings no longer mention masking, or any of the other Swiss-cheese layers of mitigation that were prevalent in the early days of this ongoing pandemic.
The problem with the prevailing, vaccine centered strategy is that if you are a parent with children under five, barriers to access are real. In many cases, meaningful tools and changes that would address these obstacles are lacking, such as offering vaccines at more locations, mandating masks at these sites, and providing paid leave time to get the shots.
Children are at risk
Data presented at the most recent FDA advisory panel on COVID-19 vaccines showed that in the last year infants under six months had the third highest rate of hospitalization. “From the beginning, the message has been that kids don’t get COVID, and then the message was, well kids get COVID, but it’s not serious,” says Elias Kass, a pediatrician in Seattle. “Then they waited so long on the initial vaccines that by the time kids could get vaccinated, the majority of them had been infected.”
A closer look at the data from the CDC also reveals that from January 2022 to January 2023 children aged 6 to 23 months were more likely to be hospitalized than all other vaccine eligible pediatric age groups.
“We sort of forced an entire generation of kids to be infected with a novel virus and just don't give a shit, like nobody cares about kids,” Kass says. In some cases, COVID has wreaked havoc with the immune systems of very young children at his practice, making them vulnerable to other illnesses, he said. “And now we have kids that have had COVID two or three times, and we don’t know what is going to happen to them.”
Jumping through hurdles
Children under five were the last group to have an emergency use authorization (EUA) granted for the COVID-19 vaccine, a year and a half after adult vaccine approval. In June 2022, 30,000 sites were initially available for children across the country. Six months later, when boosters became available, there were only 5,000.
Currently, only 3.8% of children under two have completed a primary series, according to the CDC. An even more abysmal 0.2% under two have gotten a booster.
Ariadne Labs, a health center affiliated with Harvard, is trying to understand why these gaps exist. In conjunction with Boston Children’s Hospital, they have created a vaccine equity planner that maps the locations of vaccine deserts based on factors such as social vulnerability indexes and transportation access.
“People are having to travel farther because the sites are just few and far between,” says Benjy Renton, a research assistant at Ariadne.
Michelle Baltes-Breitwisch, a pharmacist, and her two-year-old daughter, Charlee, live in Iowa. When the boosters first came out she expected her toddler could get it close to home, but her husband had to drive Charlee four hours roundtrip.
This experience hasn’t been uncommon, especially in rural parts of the U.S. If parents wanted vaccines for their young children shortly after approval, they faced the prospect of loading babies and toddlers, famous for their calm demeanor, into cars for lengthy rides. The situation continues today. Mrs. Smith*, a grant writer and non-profit advisor who lives in Idaho, is still unable to get her child the bivalent booster because a two-hour one-way drive in winter weather isn’t possible.
It can be more difficult for low wage earners to take time off, which poses challenges especially in a number of rural counties across the country, where weekend hours for getting the shots may be limited.
Protect Their Future (PTF), a grassroots organization focusing on advocacy for the health care of children, hears from parents several times a week who are having trouble finding vaccines. The vaccine rollout “has been a total mess,” says Tamara Lea Spira, co-founder of PTF “It’s been very hard for people to access vaccines for children, particularly those under three.”
Seventeen states have passed laws that give pharmacists authority to vaccinate as young as six months. Under federal law, the minimum age in other states is three. Even in the states that allow vaccination of toddlers, each pharmacy chain varies. Some require prescriptions.
It takes time to make phone calls to confirm availability and book appointments online. “So it means that the parents who are getting their children vaccinated are those who are even more motivated and with the time and the resources to understand whether and how their kids can get vaccinated,” says Tiffany Green, an associate professor in population health sciences at the University of Wisconsin at Madison.
Green adds, “And then we have the contraction of vaccine availability in terms of sites…who is most likely to be affected? It's the usual suspects, children of color, disabled children, low-income children.”
It can be more difficult for low wage earners to take time off, which poses challenges especially in a number of rural counties across the country, where weekend hours for getting the shots may be limited. In Bibb County, Ala., vaccinations take place only on Wednesdays from 1:45 to 3:00 pm.
“People who are focused on putting food on the table or stressed about having enough money to pay rent aren't going to prioritize getting vaccinated that day,” says Julia Raifman, assistant professor of health law, policy and management at Boston University. She created the COVID-19 U.S. State Policy Database, which tracks state health and economic policies related to the pandemic.
Most states in the U.S. lack paid sick leave policies, and the average paid sick days with private employers is about one week. Green says, “I think COVID should have been a wake-up call that this is necessary.”
Maskless waiting rooms
For her son, Holmes spent hours making phone calls but could uncover no clear answers. No one could estimate an arrival date for the booster. “It disappoints me greatly that the process for locating COVID-19 vaccinations for young children requires so much legwork in terms of time and resources,” she says.
In January, she found a pharmacy 30 minutes away that could vaccinate Theo. With her son being too young to mask, she waited in the car with him as long as possible to avoid a busy, maskless waiting room.
Kids under two, such as Theo, are advised not to wear masks, which make it too hard for them to breathe. With masking policies a rarity these days, waiting rooms for vaccines present another barrier to access. Even in healthcare settings, current CDC guidance only requires masking during high transmission or when treating COVID positive patients directly.
“This is a group that is really left behind,” says Raifman. “They cannot wear masks themselves. They really depend on others around them wearing masks. There's not even one train car they can go on if their parents need to take public transportation… and not risk COVID transmission.”
Yet another challenge is presented for those who don’t speak English or Spanish. According to Translators without Borders, 65 million people in America speak a language other than English. Most state departments of health have a COVID-19 web page that redirects to the federal vaccines.gov in English, with an option to translate to Spanish only.
The main avenue for accessing information on vaccines relies on an internet connection, but 22 percent of rural Americans lack broadband access. “People who lack digital access, or don’t speak English…or know how to navigate or work with computers are unable to use that service and then don’t have access to the vaccines because they just don’t know how to get to them,” Jirmanus, an affiliate of the FXB Center for Health and Human Rights at Harvard and a member of The People’s CDC explains. She sees this issue frequently when working with immigrant communities in Massachusetts. “You really have to meet people where they’re at, and that means physically where they’re at.”
Equitable solutions
Grassroots and advocacy organizations like PTF have been filling a lot of the holes left by spotty federal policy. “In many ways this collective care has been as important as our gains to access the vaccine itself,” says Spira, the PTF co-founder.
PTF facilitates peer-to-peer networks of parents that offer support to each other. At least one parent in the group has crowdsourced information on locations that are providing vaccines for the very young and created a spreadsheet displaying vaccine locations. “It is incredible to me still that this vacuum of information and support exists, and it took a totally grassroots and volunteer effort of parents and physicians to try and respond to this need.” says Spira.
Kass, who is also affiliated with PTF, has been vaccinating any child who comes to his independent practice, regardless of whether they’re one of his patients or have insurance. “I think putting everything on retail pharmacies is not appropriate. By the time the kids' vaccines were released, all of our mass vaccination sites had been taken down.” A big way to help parents and pediatricians would be to allow mixing and matching. Any child who has had the full Pfizer series has had to forgo a bivalent booster.
“I think getting those first two or three doses into kids should still be a priority, and I don’t want to lose sight of all that,” states Renton, the researcher at Ariadne Labs. Through the vaccine equity planner, he has been trying to see if there are places where mobile clinics can go to improve access. Renton continues to work with local and state planners to aid in vaccine planning. “I think any way we can make that process a lot easier…will go a long way into building vaccine confidence and getting people vaccinated,” Renton says.

Michelle Baltes-Breitwisch, a pharmacist, and her two-year-old daughter, Charlee, live in Iowa. Her husband had to drive four hours roundtrip to get the boosters for Charlee.
Michelle Baltes-Breitwisch
Other changes need to come from the CDC. Even though the CDC “has this historic reputation and a mission of valuing equity and promoting health,” Jirmanus says, “they’re really failing. The emphasis on personal responsibility is leaving a lot of people behind.” She believes another avenue for more equitable access is creating legislation for upgraded ventilation in indoor public spaces.
Given the gaps in state policies, federal leadership matters, Raifman says. With the FDA leaning toward a yearly COVID vaccine, an equity lens from the CDC will be even more critical. “We can have data driven approaches to using evidence based policies like mask policies, when and where they're most important,” she says. Raifman wants to see a sustainable system of vaccine delivery across the country complemented with a surge preparedness plan.
With the public health emergency ending and vaccines going to the private market sometime in 2023, it seems unlikely that vaccine access is going to improve. Now more than ever, ”We need to be able to extend to people the choice of not being infected with COVID,” Jirmanus says.
*Some names were changed for privacy reasons.
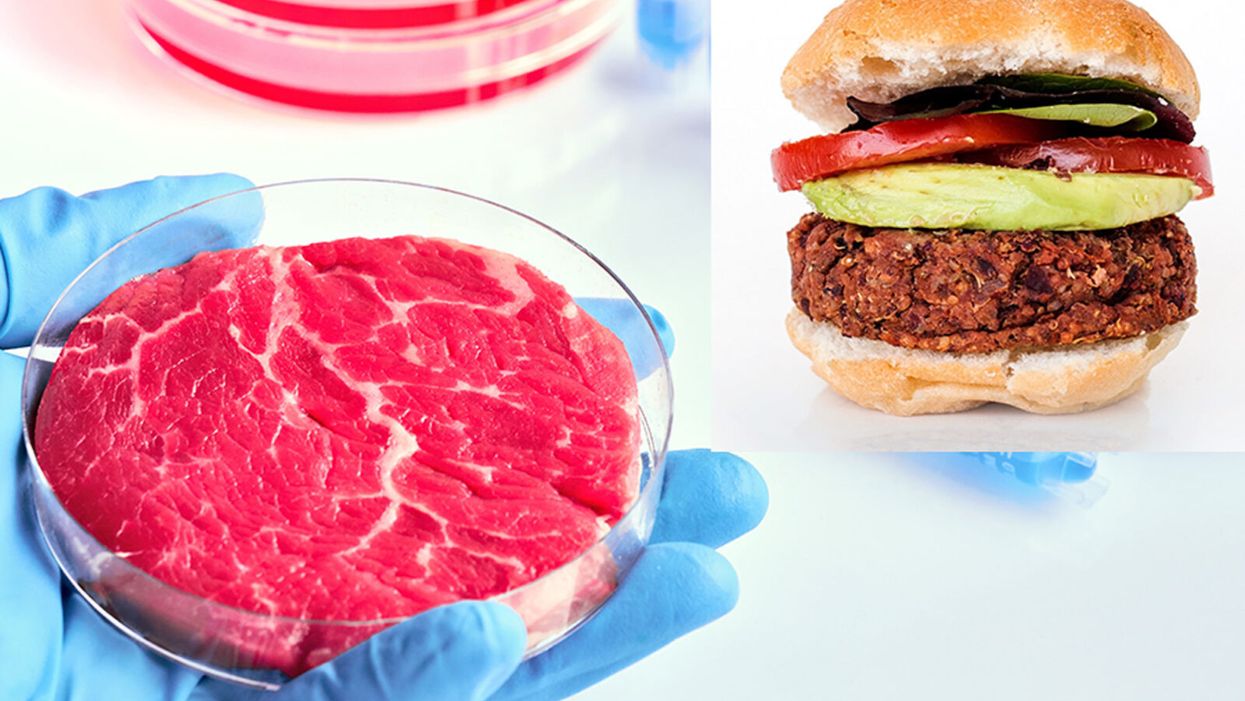
While lab-grown meat is not yet ready to crisis-proof the food supply chain, it offers key benefits that could make it an important solution beyond this pandemic.
The coronavirus pandemic exposed significant weaknesses in the country's food supply chain. Grocery store meat counters were bare. Transportation interruptions influenced supply. Finding beef, poultry, and pork at the store has been, in some places, as challenging as finding toilet paper.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle.
It wasn't a lack of supply -- millions of animals were in the pipeline.
"There's certainly enough food out there, but it can't get anywhere because of the way our system is set up," said Amy Rowat, an associate professor of integrative biology and physiology at UCLA. "Having a more self-contained, self-sufficient way to produce meat could make the supply chain more robust."
Cultured meat could be one way of making the meat supply chain more resilient despite disruptions due to pandemics such as COVID-19. But is the country ready to embrace lab-grown food?
According to a Good Food Institute study, GenZ is almost twice as likely to embrace meat alternatives for reasons related to social and environmental awareness, even prior to the pandemic. That's because this group wants food choices that reflect their values around food justice, equity, and animal welfare.
Largely, the interest in protein alternatives has been plant-based foods. However, factors directly related to COVID-19 may accelerate consumer interest in the scaling up of cell-grown products, according to Liz Specht, the associate director of science and technology at The Good Food Institute. The latter is a nonprofit organization that supports scientists, investors, and entrepreneurs working to develop food alternatives to conventional animal products.
While lab-grown food isn't ready yet to definitively crisis-proof the food supply chain, experts say it offers promise.
Matching Supply and Demand
Companies developing cell-grown meat claim it can take as few as two months to develop a cell into an edible product, according to Anthony Chow, CFA at Agronomics Limited, an investment company focused on meat alternatives. Tissue is taken from an animal and placed in a culture that contains nutrients and proteins the cells need to grow and expand. He cites a Good Food Institute report that claims a 2.5-millimeter sample can grow three and a half tons of meat in 40 days, allowing for exponential growth when needed.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle. To keep enough maturing animals in the pipeline, farms must plan the number of animals to raise months -- even years -- in advance. Lab-grown meat advocates say that because cultured meat supplies can be flexible, it theoretically allows for scaling up or down in significantly less time.
"Supply and demand has drastically changed in some way around the world and cultivated meat processing would be able to adapt much quicker than conventional farming," Chow said.
Scaling Up
Lab-grown meat may provide an eventual solution, but not in the immediate future, said Paul Mozdziak, a professor of physiology at North Carolina State University who researches animal cell culture techniques, transgenic animal production, and muscle biology.
"The challenge is in culture media," he said. "It's going to take some innovation to get the cells to grow at quantities that are going to be similar to what you can get from an animal. These are questions that everybody in the space is working on."
Chow says some of the most advanced cultured meat companies, such as BlueNal, anticipate introducing products to the market midway through next year. However, he thinks COVID-19 has slowed the process. Once introduced, they will be at a premium price, most likely available at restaurants before they hit grocery store shelves.
"I think in five years' time it will be in a different place," he said. "I don't think that this will have relevance for this pandemic, but certainly beyond that."
"Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
Of course, all the technological solutions in the world won't solve the problem unless people are open-minded about embracing them. At least for now, a lab-grown burger or bluefin tuna might still be too strange for many people, especially in the U.S.
For instance, a 2019 article published by "Frontiers in Sustainable Food Systems" reflects results from a study of 3,030 consumers showing that 29 percent of U.S. customers, 59 percent of Chinese consumers, and 56 percent of Indian consumers were either 'very' or 'extremely likely' to try cultivated meat.
"Lab-grown meat is genuine meat, at the cellular level, and therefore will match conventional meat with regard to its nutritional content and overall sensory experience. It could be argued that plant-based meat will never be able to achieve this," says Laura Turner, who works with Chow at Agronomics Limited. "Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
A Solution Beyond This Pandemic
The coronavirus has done more than raise awareness of the fragility of food supply chains. It has also been a wakeup call for consumers and policy makers that it is time to radically rethink our meat, Specht says. Those factors have elevated the profile of lab-grown meat.
"I think the economy is getting a little bit more steam and if I was an investor, I would be getting excited about it," adds Mozdziak.
Beyond crises, Mozdziak explains that as affluence continues to increase globally, meat consumption increases exponentially. Yet farm animals can only grow so quickly and traditional farming won't be able to keep up.
"Even Tyson is saying that by 2050, there's not going to be enough capacity in the animal meat space to meet demand," he notes. "If we don't look at some innovative technologies, how are we going to overcome that?"
A COVID-19 moonshot program challenged chemists around the world to submit ideas for how best to design a drug to target the virus.
By mid-March, Alpha Lee was growing restless. A pioneer of AI-driven drug discovery, Lee leads a team of researchers at the University of Cambridge, but his lab had been closed amidst the government-initiated lockdowns spreading inexorably across Europe.
If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Having spoken to his collaborators across the globe – many of whom were seeing their own experiments and research projects postponed indefinitely due to the pandemic – he noticed a similar sense of frustration and helplessness in the face of COVID-19.
While there was talk of finding a novel treatment for the virus, Lee was well aware the process was likely to be long and laborious. Traditional methods of drug discovery risked suffering the same fate as the efforts to find a cure for SARS in the early 2000, which took years and were ultimately abandoned long before a drug ever reached the market.
To avoid such an outcome, Lee was convinced that global collaboration was required. Together with a collection of scientists in the UK, US and Israel, he launched the 'COVID Moonshot' – a project which encouraged chemists worldwide to share their ideas for potential drug designs. If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Solving a Complex Jigsaw
In February, ShanghaiTech University published the first detailed snapshots of the SARS-CoV-2 coronavirus's proteins using a technique called X-ray crystallography. In particular, they revealed a high-resolution profile of the virus's main protease – the part of its structure that enables it to replicate inside a host – and the main drug target. The images were tantalizing.
"We could see all the tiny pieces sitting in the structure like pieces of a jigsaw," said Lee. "All we needed was for someone to come up with the best idea of joining these pieces together with a drug. Then you'd be left with a strong molecule which sits in the protease, and stops it from working, killing the virus in the process."
Normally, ideas for how best to design such a drug would be kept as carefully guarded secrets within individual labs and companies due to their potential value. But as a result, the steady process of trial and error to reach an optimum design can take years to come to fruition.
However, given the scale of the global emergency, Lee felt that the scientific community would be open to collective brainstorming on a mass scale. "Big Pharma usually wouldn't necessarily do this, but time is of the essence here," he said. "It was a case of, 'Let's just rethink every drug discovery stage to see -- ok, how can we go as fast as we can?'"
On March 13, he launched the COVID moonshot, calling for chemists around the globe to come up with the most creative ideas they could think of, on their laptops at home. No design was too weird or wacky to be considered, and crucially nothing would be patented. The entire project would be done on a not-for-profit basis, meaning that any drug that makes it to market will have been created simply for the good of humanity.
It caught fire: Within just two weeks, more than 2,300 potential drug designs had been submitted. By the middle of July, over 10,000 had been received from scientists around the globe.
The Road Toward Clinical Trials
With so many designs to choose from, the team has been attempting to whittle them down to a shortlist of the most promising. Computational drug discovery experts at Diamond and the Weizmann Institute of Science in Rehovot, Israel, have enabled the Moonshot team to develop algorithms for predicting how quick and easy each design would be to make, and to predict how well each proposed drug might bind to the virus in real life.
The latter is an approach known as computational covalent docking and has previously been used in cancer research. "This was becoming more popular even before COVID-19, with several covalent drugs approved by the FDA in recent years," said Nir London, professor of organic chemistry at the Weizmann Institute, and one of the Moonshot team members. "However, all of these were for oncology. A covalent drug against SARS-CoV-2 will certainly highlight covalent drug-discovery as a viable option."
Through this approach, the team have selected 850 compounds to date, which they have manufactured and tested in various preclinical trials already. Fifty of these compounds - which appear to be especially promising when it comes to killing the virus in a test tube – are now being optimized further.
Lee is hoping that at least one of these potential drugs will be shown to be effective in curing animals of COVID-19 within the next six months, a step that would allow the Moonshot team to reach out to potential pharmaceutical partners to test their compounds in humans.
Future Implications
If the project does succeed, some believe it could open the door to scientific crowdsourcing as a future means of generating novel medicine ideas for other diseases. Frank von Delft, professor of protein science and structural biology at the University of Oxford's Nuffield Department of Medicine, described it as a new form of 'citizen science.'
"There's a vast resource of expertise and imagination that is simply dying to be tapped into," he said.
Others are slightly more skeptical, pointing out that the uniqueness of the current crisis has meant that many scientists were willing to contribute ideas without expecting any future compensation in return. This meant that it was easy to circumvent the traditional hurdles that prevent large-scale global collaborations from happening – namely how to decide who will profit from the final product and who will hold the intellectual property (IP) rights.
"I think it is too early to judge if this is a viable model for future drug discovery," says London. "I am not sure that without the existential threat we would have seen so many contributions, and so many people and institutions willing to waive compensation and future royalties. Many scientists found themselves at home, frustrated that they don't have a way to contribute to the fight against COVID-19, and this project gave them an opportunity. Plus many can get behind the fact that this project has no associated IP and no one will get rich off of this effort. This breaks down a lot of the typical barriers and red-tape for wider collaboration."
"If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
However the Moonshot team believes that if they can succeed, it will at the very least send a strong statement to policy makers and the scientific community that greater efforts should be made to make such large-scale collaborations more feasible.
"All across the scientific world, we've seen unprecedented adoption of open-science, collaboration and collegiality during this crisis, perhaps recognizing that only a coordinated global effort could address this global challenge," says London. "If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
[An earlier version of this article was published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]