Blood Money: Paying for Convalescent Plasma to Treat COVID-19
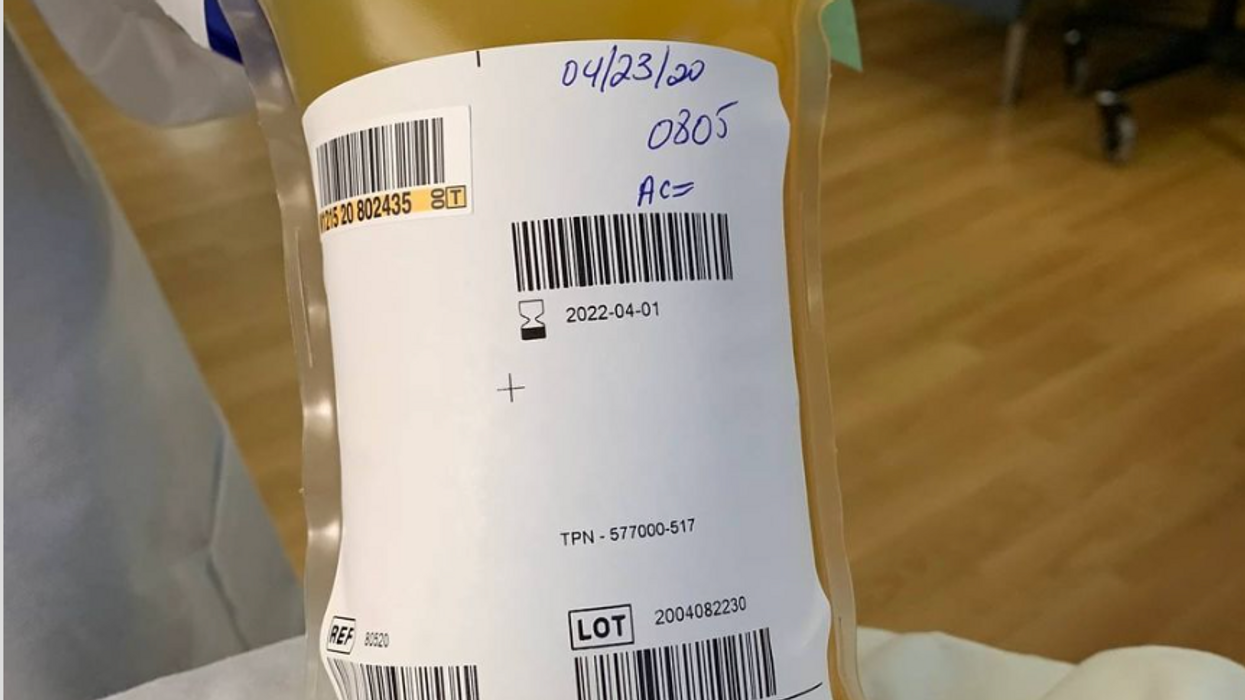
A bag of plasma that Tom Hanks donated back in April 2020 after his coronavirus infection. (He was not paid to donate.)
Convalescent plasma – first used to treat diphtheria in 1890 – has been dusted off the shelf to treat COVID-19. Does it work? Should we rely strictly on the altruism of donors or should people be paid for it?
The biologic theory is that a person who has recovered from a disease has chemicals in their blood, most likely antibodies, that contributed to their recovery, and transferring those to a person who is sick might aid their recovery. Whole blood won't work because there are too few antibodies in a single unit of blood and the body can hold only so much of it.
Plasma comprises about 55 percent of whole blood and is what's left once you take out the red blood cells that carry oxygen and the white blood cells of the immune system. Most of it is water but the rest is a complex mix of fats, salts, signaling molecules and proteins produced by the immune system, including antibodies.
A process called apheresis circulates the donors' blood through a machine that separates out the desired parts of blood and returns the rest to the donor. It takes several times the length of a regular whole blood donation to cycle through enough blood for the process. The end product is a yellowish concentration called convalescent plasma.
Recent History
It was used extensively during the great influenza epidemic off 1918 but fell out of favor with the development of antibiotics. Still, whenever a new disease emerges – SARS, MERS, Ebola, even antibiotic-resistant bacteria – doctors turn to convalescent plasma, often as a stopgap until more effective antibiotic and antiviral drugs are developed. The process is certainly safe when standard procedures for handling blood products are followed, and historically it does seem to be beneficial in at least some patients if administered early enough in the disease.
With few good treatment options for COVID-19, doctors have given convalescent plasma to more than a hundred thousand Americans and tens of thousand of people elsewhere, to mixed results. Placebo-controlled trials could give a clearer picture of plasma's value but it is difficult to enroll patients facing possible death when the flip of a coin will determine who will receive a saline solution or plasma.
And the plasma itself isn't some uniform pill stamped out in a factory, it's a natural product that is shaped by the immune history of the donor's body and its encounter not just with SARS-CoV-2 but a lifetime of exposure to different pathogens.
Researchers believe antibodies in plasma are a key factor in directly fighting the virus. But the variety and quantity of antibodies vary from donor to donor, and even over time from the same donor because once the immune system has cleared the virus from the body, it stops putting out antibodies to fight the virus. Often the quality and quantity of antibodies being given to a patient are not measured, making it somewhat hit or miss, which is why several companies have recently developed monoclonal antibodies, a single type of antibody found in blood that is effective against SARS-CoV-2 and that is multiplied in the lab for use as therapy.
Plasma may also contain other unknown factors that contribute to fighting disease, say perhaps signaling molecules that affect gene expression, which might affect the movement of immune cells, their production of antiviral molecules, or the regulation of inflammation. The complexity and lack of standardization makes it difficult to evaluate what might be working or not with a convalescent plasma treatment. Thus researchers are left with few clues about how to make it more effective.
Industrializing Plasma
Many Americans living along the border with Mexico regularly head south to purchase prescription drugs at a significant discount. Less known is the medical traffic the other way, Mexicans who regularly head north to be paid for plasma donations, which are prohibited in their country; the U.S. allows payment for plasma donations but not whole blood. A typical payment is about $35 for a donation but the sudden demand for convalescent plasma from people who have recovered from COVID-19 commands a premium price, sometimes as high as $200. These donors are part of a fast-growing plasma industry that surpassed $25 billion in 2018. The U.S. supplies about three-quarters of the world's needs for plasma.
Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free.
The pharmaceutical industry has shied away from natural products they cannot patent but they have identified simpler components from plasma, such as clotting factors and immunoglobulins, that have been turned into useful drugs from this raw material of plasma. While some companies have retooled to provide convalescent plasma to treat COVID-19, often paying those donors who have recovered a premium of several times the normal rate, most convalescent plasma has come as donations through traditional blood centers.
In April the Mayo Clinic, in cooperation with the FDA, created an expanded access program for convalescent plasma to treat COVID-19. It was meant to reduce the paperwork associated with gaining access to a treatment not yet approved by the FDA for that disease. Initially it was supposed to be for 5000 units but it quickly grew to more than twenty times that size. Michael Joyner, the head of the program, discussed that experience in an extended interview in September.
The Centers for Medicare and Medicaid Services (CMS) also created associated reimbursement codes, which became permanent in August.
Mayo published an analysis of the first 35,000 patients as a preprint in August. It concluded, "The relationships between mortality and both time to plasma transfusion, and antibody levels provide a signature that is consistent with efficacy for the use of convalescent plasma in the treatment of hospitalized COVID-19 patients."
It seemed to work best when given early in infection and in larger doses; a similar pattern has been seen in studies of monoclonal antibodies. A revised version will soon be published in a major medical journal. Some criticized the findings as not being from a randomized clinical trial.
Convalescent plasma is not the only intervention that seems to work better when used earlier in the course of disease. Recently the pharmaceutical company Eli Lilly stopped a clinical trial of a monoclonal antibody in hospitalized COVID-19 patients when it became apparent it wasn't helping. It is continuing trials for patients who are less sick and begin treatment earlier, as well as in persons who have been exposed to the virus but not yet diagnosed as infected, to see if it might prevent infection. In November the FDA eased access to this drug outside of clinical trials, though it is not yet approved for sale.
Show Me the Money
The antibodies that seem to give plasma its curative powers are fragile proteins that the body produces to fight the virus. Production shuts down once the virus is cleared and the remaining antibodies survive only for a few weeks before the levels fade. [Vaccines are used to train immune cells to produce antibodies and other defenses to respond to exposure to future pathogens.] So they can be usefully harvested from a recovered patient for only a few short weeks or months before they decline precipitously. The question becomes, how does one mobilize this resource in that short window of opportunity?
The program run by the Mayo Clinic explains the process and criteria for donating convalescent plasma for COVID-19, as well as links to local blood centers equipped to handle those free donations. Commercial plasma centers also are advertising and paying for donations.
A majority of countries prohibit paying donors for blood or blood products, including India. But an investigation by India Today touted a black market of people willing to donate convalescent plasma for the equivalent of several hundred dollars. Officials vowed to prosecute, saying donations should be selfless.
But that enforcement threat seemed to be undercut when the health minister of the state of Assam declared "plasma donors will get preference in several government schemes including the government job interview." It appeared to be a form of compensation that far surpassed simple cash.
The small city of Rexburg, Idaho, with a population a bit over 50,000, overwhelmingly Mormon and home to a campus of Brigham Young University, at one point had one of the highest per capita rates of COVID-19 in the current wave of infection. Rumors circulated that some students were intentionally trying to become infected so they could later sell their plasma for top dollar, potentially as much as $200 a visit.
Troubled university officials investigated the allegations but could come up with nothing definitive; how does one prove intentionality with such an omnipresent yet elusive virus? They chalked it up to idle chatter, perhaps an urban legend, which might be associated with alcohol use on some other campus.
Doctors, hospitals, and drug companies are all rightly praised for their altruism in the fight against COVID-19, but they also get paid. Payment for whole blood donation in the U.S. is prohibited, and while payment for plasma is allowed, there is a stigma attached to payment and much plasma is donated for free. "Why do we expect the donors [of convalescent plasma] to be the only uncompensated people in the process? It really makes no sense," argues Mark Yarborough, an ethicist at the UC Davis School of Medicine in Sacramento.
"When I was in grad school, two of my closest friends, at least once a week they went and gave plasma. That was their weekend spending money," Yarborough recalls. He says upper and middle-income people may have the luxury of donating blood products but prohibiting people from selling their plasma is a bit paternalistic and doesn't do anything to improve the economic status of poor people.
"Asking people to dedicate two hours a week for an entire year in exchange for cookies and milk is demonstrably asking too much," says Peter Jaworski, an ethicist who teaches at Georgetown University.
He notes that companies that pay plasma donors have much lower total costs than do operations that rely solely on uncompensated donations. The companies have to spend less to recruit and retain donors because they increase payments to encourage regular repeat donations. They are able to more rationally schedule visits to maximize use of expensive apheresis equipment and medical personnel used for the collection.
It seems that COVID-19 has been with us forever, but in reality it is less than a year. We have learned much over that short time, can now better manage the disease, and have lower mortality rates to prove it. Just how much convalescent plasma may have contributed to that remains an open question. Access to vaccines is months away for many people, and even then some people will continue to get sick. Given the lack of proven treatments, it makes sense to keep plasma as part of the mix, and not close the door to any legitimate means to obtain it.
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

