A New Stem Cell Therapy Provides Hope to Patients with Blood Cancer
Stacey Khoury felt more fatigued and out of breath than she was used to from just walking up the steps to her job in retail jewelry sales in Nashville, Tennessee. By the time she got home, she was more exhausted than usual, too.
"I just thought I was working too hard and needed more exercise," recalls the native Nashvillian about those days in December 2010. "All of the usual excuses you make when you're not feeling 100%."
As a professional gemologist, being hospitalized during peak holiday sales season wasn't particularly convenient. There was no way around it though when her primary care physician advised Khoury to see a blood disorder oncologist because of her disturbing blood count numbers. As part of a routine medical exam, a complete blood count screens for a variety of diseases and conditions that affect blood cells, such as anemia, infection, inflammation, bleeding disorders and cancer.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before."
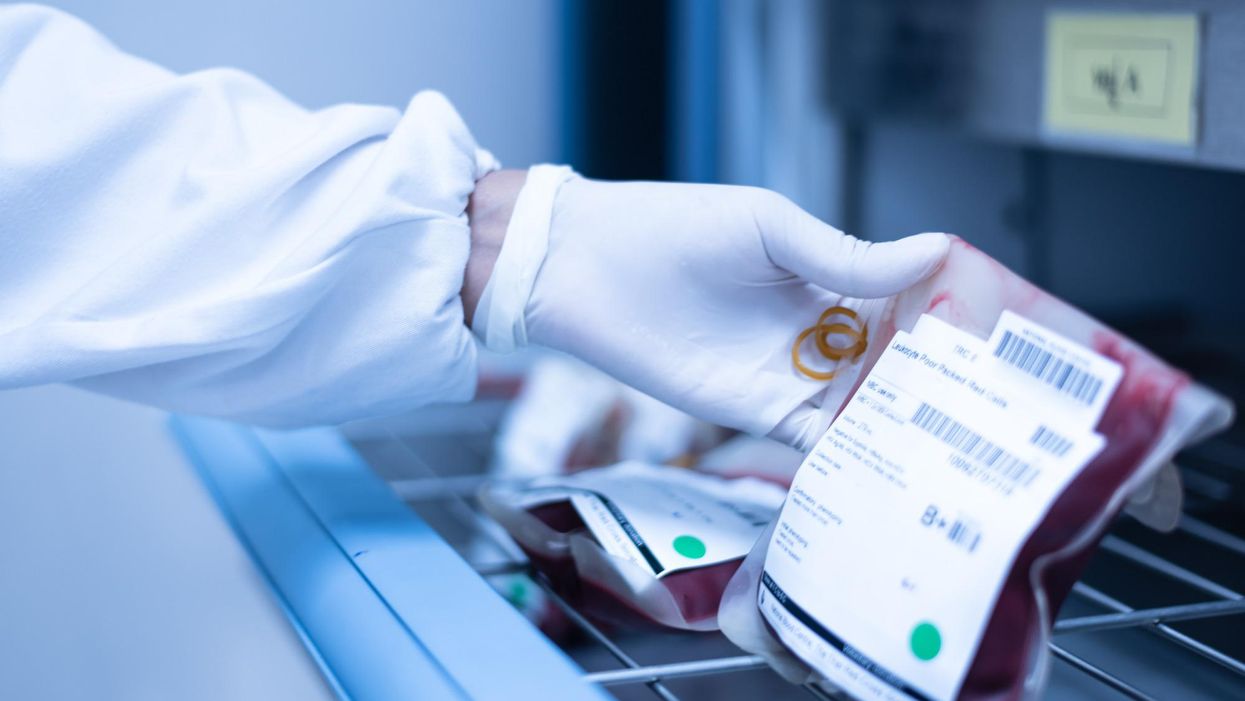
While she was in the hospital, a bone marrow biopsy revealed that Khoury had acute myeloid leukemia, or AML, a high-risk blood cancer. After Khoury completed an intense first round of chemotherapy, her oncologist recommended a bone marrow transplant. The potentially curative treatment for blood-cancer patients requires them to first receive a high dose of chemotherapy. Next, an infusion of stem cells from a healthy donor's bone marrow helps form new blood cells to fight off the cancer long-term.
Each year, approximately 8,000 patients in the U.S. with AML and other blood cancers receive a bone marrow transplant from a donor, according to the Center for International Blood and Marrow Transplant Research. But Khoury wasn't so lucky. She ended up being among the estimated 40% of patients eligible for bone marrow transplants who don't receive one, usually because there's no matched donor available.
Khoury's oncologist told her about another option. She could enter a clinical trial for an investigational cell therapy called omidubicel, which is being developed by Israeli biotech company Gamida Cell. The company's cell therapy, which is still experimental, could up a new avenue of treatment for cancer patients who can't get a bone marrow transplant.
Omidubicel consists of stem cells from cord blood that have been expanded using Gamida's technology to ensure there are enough cells for a therapeutic dose. The company's technology allows the immature cord blood cells to multiply quickly in the lab. Like a bone marrow transplant, the goal of the therapy is to make sure the donor cells make their way to the bone marrow and begin producing healthy new cells — a process called engraftment.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before, so there's something very novel and exciting about that," says Ronit Simantov, Gamida Cell's chief medical officer.
Khoury and her husband Rick packed up their car and headed to the closest trial site, the Duke University School of Medicine, roughly 500 miles away. There they met with Mitchell Horowitz, a stem cell transplant specialist at Duke and principal investigator for Gamida's omidubicel study in the U.S.
He told Khoury she was a perfect candidate for the trial, and she enrolled immediately. "When you have one of two decisions, and it's either do this or you're probably not going to be around, it was a pretty easy decision to make, and I am truly thankful for that," she says.
Khoury's treatment started at the end of March 2011, and she was home by July 4 that year. She say the therapy "worked the way the doctors wanted it to work." Khoury's blood counts were rising quicker than the people who had bone marrow matches, and she was discharged from Duke earlier than other patients were.
By expanding the number of cord blood cells — which are typically too few to treat an adult — omidubicel allows doctors to use cord blood for patients who require a transplant but don't have a donor match for bone marrow.
Patients receiving omidubicel first get a blood test to determine their human leukocyte antigen, or HLA, type. This protein is found on most cells in the body and is an important regulator of the immune system. HLA typing is used to match patients to bone marrow and cord blood donors, but cord blood doesn't require as close of a match.
Like bone marrow transplants, one potential complication of omidubicel is graft-versus-host disease, when the donated bone marrow or stem cells register the recipient's body as foreign and attack the body. Depending on the severity of the response, according to the Mayo Clinic, treatment includes medication to suppress the immune system, such as steroids. In clinical trials, the occurrence of graft-versus-host disease with omidubicel was comparable with traditional bone marrow transplants.
"Transplant doctors are working on improving that," Simantov says. "A number of new therapies that specifically address graft-versus-host disease will be making some headway in the coming months and years."
Gamida released the results of the Phase 3 study in February and continues to follow Khoury and the other study patients for their long-term outcomes. The large randomized trial evaluated the safety and efficacy of omidubicel compared to standard umbilical cord blood transplants in patients with blood cancer who didn't have a suitable bone marrow donor. Around 120 patients aged 12 to 65 across the U.S., Europe and Asia were included in the trial. The study found that omidubicel resulted in faster recovery, fewer bacterial and viral infections and fewer days in the hospital.
The company plans to seek FDA approval this year. Simantov anticipates the therapy will receive FDA approval by 2022.
"Opening up cord blood transplants is very important, especially for people of diverse ethnic backgrounds," says oncologist Gary Schiller, principal investigator at the David Geffen School of Medicine at UCLA for Gamida Cell's mid- and late-stage trials. "This expansion technology makes a big difference because it makes cord blood an available option for those who do not have another donor source."
As for Khoury, who proudly celebrated the anniversary of her first transplant in April—she remains cancer free and continues to work full-time as a gemologist. When she has a little free time, she enjoys gardening, sewing, or maybe traveling to national parks like Yellowstone or the Grand Canyon with her husband Rick.
Scientists are making progress to create a one-time therapy that would permanently lower LDL cholesterol to prevent heart attacks caused by high LDL.
Later this year, Verve Therapeutics of Cambridge, Ma., will initiate Phase 1 clinical trials to test VERVE-101, a new medication that, if successful, will employ gene editing to significantly reduce low-density lipoprotein cholesterol, or LDL.
LDL is sometimes referred to as the “bad” cholesterol because it collects in the walls of blood vessels, and high levels can increase chances of a heart attack, cardiovascular disease or stroke. There are approximately 600,000 heart attacks per year due to blood cholesterol damage in the United States, and heart disease is the number one cause of death in the world. According to the CDC, a 10 percent decrease in total blood cholesterol levels can reduce the incidence of heart disease by as much as 30 percent.
Verve’s Founder and CEO, Sekar Kathiresan, spent two decades studying the genetic basis for heart attacks while serving as a professor of medicine at Harvard Medical School. His research led to two critical insights.
“One is that there are some people that are naturally resistant to heart attack and have lifelong, low levels of LDL,” the cardiologist says. “Second, there are some genes that can be switched off that lead to very low LDL cholesterol, and individuals with those genes switched off are resistant to heart attacks.”
Kathiresan and his team formed a hypothesis in 2016 that if they could develop a medicine that mimics the natural protection that some people enjoy, then they might identify a powerful new way to treat and ultimately prevent heart attacks. They launched Verve in 2018 with the goal of creating a one-time therapy that would permanently lower LDL and eliminate heart attacks caused by high LDL.
"Imagine a future where somebody gets a one-time treatment at the time of their heart attack or before as a preventive measure," says Kathiresan.
The medication is targeted specifically for patients who have a genetic form of high cholesterol known as heterozygous familial hypercholesterolemia, or FH, caused by expression of a gene called PCSK9. Verve also plans to develop a program to silence a gene called ANGPTL3 for patients with FH and possibly those with or at risk of atherosclerotic cardiovascular disease.
FH causes cholesterol to be high from birth, reaching levels of 200 to 300 milligrams per deciliter. Suggested normal levels are around 100 to 129 mg/dl, and anything above 130 mg/dl is considered high. Patients with cardiovascular disease usually are asked to aim for under 70 mg/dl, but many still have unacceptably high LDL despite taking oral medications such as statins. They are more likely to have heart attacks in their 30s, 40s and 50s, and require lifelong LDL control.
The goal for drug treatments for high LDL, Kathiresan says, is to reduce LDL as low as possible for as long as possible. Physicians and researchers also know that a sizeable portion of these patients eventually start to lose their commitment to taking their statins and other LDL-controlling medications regularly.
“If you ask 100 patients one year after their heart attack what fraction are still taking their cholesterol-lowering medications, it’s less than half,” says Kathiresan. “So imagine a future where somebody gets a one-time treatment at the time of their heart attack or before as a preventive measure. It’s right in front of us, and it’s something that Verve is looking to do.”
In late 2020, Verve completed primate testing with monkeys that had genetically high cholesterol, using a one-time intravenous injection of VERVE-101. It reduced the monkeys’ LDL by 60 percent and, 18 months later, remains at that level. Kathiresan expects the LDL to stay low for the rest of their lives.
Verve’s gene editing medication is packaged in a lipid nanoparticle to serve as the delivery mechanism into the liver when infused intravenously. The drug is absorbed and makes its way into the nucleus of the liver cells.
Verve’s program targeting PCSK9 uses precise, single base, pair base editing, Kathiresan says, meaning it doesn't cut DNA like CRISPR gene editing systems do. Instead, it changes one base, or letter, in the genome to a different one without affecting the letters around it. Comparing it to a pencil and eraser, he explains that the medication erases out a letter A and makes it a letter G in the A, C, G and T code in DNA.
“We need to continue to advance our approach and tools to make sure that we have the absolute maximum ability to detect off-target effects,” says Euan Ashley, professor of medicine and genetics at Stanford University.
By making that simple change from A to G, the medication switches off the PCSK9 gene, automatically lowering LDL cholesterol.
“Once the DNA change is made, all the cells in the liver will have that single A to G change made,” Kathiresan says. “Then the liver cells divide and give rise to future liver cells, but every time the cell divides that change, the new G is carried forward.”
Additionally, Verve is pursuing its second gene editing program to eliminate ANGPTL3, a gene that raises both LDL and blood triglycerides. In 2010, Kathiresan's research team learned that people who had that gene completely switched off had LDL and triglyceride levels of about 20 and were very healthy with no heart attacks. The goal of Verve’s medication will be to switch off that gene, too, as an option for additional LDL or triglyceride lowering.
“Success with our first drug, VERVE-101, will give us more confidence to move forward with our second drug,” Kathiresan says. “And it opens up this general idea of making [genomic] spelling changes in the liver to treat other diseases.”
The approach is less ethically concerning than other gene editing technologies because it applies somatic editing that affects only the individual patient, whereas germline editing in the patient’s sperm or egg, or in an embryo, gets passed on to children. Additionally, gene editing therapies receive the same comprehensive amount of testing for side effects as any other medicine.
“We need to continue to advance our approach and tools to make sure that we have the absolute maximum ability to detect off-target effects,” says Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease. Ashley and his colleagues at Stanford’s Clinical Genomics Program and beyond are increasingly excited about the promise of gene editing.
“We can offer precision diagnostics, so increasingly we’re able to define the disease at a much deeper level using molecular tools and sequencing,” he continues. “We also have this immense power of reading the genome, but we’re really on the verge of taking advantage of the power that we now have to potentially correct some of the variants that we find on a genome that contribute to disease.”
He adds that while the gene editing medicines in development to correct genomes are ahead of the delivery mechanisms needed to get them into the body, particularly the heart and brain, he’s optimistic that those aren’t too far behind.
“It will probably take a few more years before those next generation tools start to get into clinical trials,” says Ashley, whose book, The Genome Odyssey, was published last year. “The medications might be the sexier part of the research, but if you can’t get it into the right place at the right time in the right dose and not get it to the places you don’t want it to go, then that tool is not of much use.”
Medical experts consider knocking out the PCSK9 gene in patients with the fairly common genetic disorder of familial hypercholesterolemia – roughly one in 250 people – a potentially safe approach to gene editing and an effective means of significantly lowering their LDL cholesterol.

Nurse Erin McGlennon has an Implantable Cardioverter Defibrillator and takes medications, but she is also hopeful that a gene editing medication will be developed in the near future.
Erin McGlennon
Mary McGowan, MD, chief medical officer for The Family Heart Foundation in Pasadena, CA, sees the tremendous potential for VERVE-101 and believes patients should be encouraged by the fact that this kind of research is occurring and how much Verve has accomplished in a relatively short time. However, she offers one caveat, since even a 60 percent reduction in LDL won’t completely eliminate the need to reduce the remaining amount of LDL.
“This technology is very exciting,” she said, “but we want to stress to our patients with familial hypercholesterolemia that we know from our published research that most people require several therapies to get their LDL down., whether that be in primary prevention less than 100 mg/dl or secondary prevention less than 70 mg/dl, So Verve’s medication would be an add-on therapy for most patients.”
Dr. Kathiresan concurs: “We expect our medicine to lower LDL cholesterol by about 60 percent and that our patients will be on background oral medications, including statins that lower LDL cholesterol.”
Several leading research centers are investigating gene editing treatments for other types of cardiovascular diseases. Elizabeth McNally, Elizabeth Ward Professor and Director at the Center for Genetic Medicine at Northwestern University’s Feinberg School of Medicine, pursues advanced genetic correction in neuromuscular diseases such as Duchenne muscular dystrophy and spinal muscular atrophy. A cardiologist, she and her colleagues know these diseases frequently have cardiac complications.
“Even though the field is driven by neuromuscular specialists, it’s the first therapies in patients with neuromuscular diseases that are also expected to make genetic corrections in the heart,” she says. “It’s almost like an afterthought that we’re potentially fixing the heart, too.”
Another limitation McGowan sees is that too many healthcare providers are not yet familiar with how to test patients to determine whether or not they carry genetic mutations that need to be corrected. “We need to get more genetic testing done,” she says. “For example, that’s the case with hypertrophic cardiomyopathy, where a lot of the people who probably carry that diagnosis and have never been genetically identified at a time when genetic testing has never been easier.”
One patient who has been diagnosed with hypertrophic cardiomyopathy also happens to be a nurse working in research at Genentech Pharmaceutical, now a member of the Roche Group, in South San Francisco. To treat the disease, Erin McGlennon, RN, has an Implantable Cardioverter Defibrillator and takes medications, but she is also hopeful that a gene editing medication will be developed in the near future.
“With my condition, the septum muscles are just growing thicker, so I’m on medicine to keep my heart from having dangerous rhythms,” says McGlennon of the disease that carries a low risk of sudden cardiac death. “So, the possibility of having a treatment option that can significantly improve my day-to-day functioning would be a major breakthrough.”
McGlennon has some control over cardiovascular destiny through at least one currently available technology: in vitro fertilization. She’s going through it to ensure that her children won't express the gene for hypertrophic cardiomyopathy.
Researchers have found that a mindfulness-based therapy, including savoring natural rewards, can help patients with chronic pain and opioid addiction.
More than 20 percent of American adults suffer from chronic pain. And as many as one in four of those prescribed opioids to manage that pain go on to misuse – or abuse – them, often with devastating consequences. Patients afflicted by both chronic pain and opioid addiction are especially difficult to treat, according to Eric Garland, PhD, Director of the University of Utah’s Center on Mindfulness and Integrative Health Intervention Development, because opioid overuse increases pain sensitivity, and pain promotes relapse among those being treated for addiction.
A new study, however, shows that a mindfulness-based therapy can successfully tackle both problems at once, pointing to a tool that could potentially help in fighting the opioid crisis. “This is the first large-scale clinical trial to show that any psychological intervention can reduce opioid misuse and chronic pain for the long term,” says Garland, lead author of the study, published February 28th in JAMA Internal Medicine.
Garland’s study focused on 250 adults who had received opioid therapy for chronic pain for 90 days or longer, randomly assigning them to eight weeks of either a standard psychotherapy support group or Mindfulness-Oriented Recovery Enhancement (MORE) therapy, which combines mindfulness training, cognitive-behavioral therapy (CBT) and positive psychology. Nine months after getting these treatments in primary care settings, 45 percent of patients in the MORE group were no longer misusing opioids, compared to 24 percent of those in group therapy. In fact, about a third of the patients in the MORE group were able to cut their opioid dose in half or reduce it even further.
Patients treated with MORE also experienced more significant pain relief than those in support groups, according to Garland. Conventional approaches to treating opioid addiction include 12-step programs and medically-assisted treatment using drugs like methadone and Suboxone, sometimes coupled with support groups. But patients with Opioid Use Disorder (OUD) – the official diagnosis for opioid addiction – have high relapse rates following treatment, especially if they have chronic pain.
While medically-assisted treatments help to control drug cravings, they do nothing to control chronic pain, which is where psychological therapies like MORE come in.
“For patients suffering from moderate pain and OUD, the relapse rate is three times higher than in patients without chronic pain; for those with severe chronic pain, the relapse rate is five times higher,” says Amy Wachholtz, PhD, Director of Clinical Health Psychology and associate professor at University of Colorado in Denver. “So if we don’t treat the chronic pain along with the OUD addiction simultaneously, we are setting patients up for failure.”
Unfortunately, notes Garland, the standard of care for patients with chronic pain who are misusing their prescribed painkillers is “woefully inadequate.” Many patients don’t meet the criteria for OUD, he says, but instead fall into a gray zone somewhere between legitimate opioid use and full-blown addiction. And while medically-assisted treatments help to control drug cravings, they do nothing to control chronic pain, which is where psychological therapies like MORE come in. But behavioral therapies are often not available in primary care settings, and even when clinicians do refer patients to behavioral health providers, they often prescribe CBT. A large scale study last year showed that CBT – without the added components of mindfulness training and positive psychology – reduced pain but not opioid misuse.

Psychotherapist Eric Garland teaches mindfulness.
University of Utah
Reward Circuitry Rewired
Opioids are highly physiologically addictive. Repeated and high-dose drug use causes the brain to become hypersensitive to stress, pain, and drug-related cues, such as the sight of one’s pill bottle, says Garland, while at the same time becoming increasingly insensitive to natural pleasures. “As an individual becomes more and more dependent on the opioids just to feel okay, they feel less able to extract a healthy sense of joy, pleasure and meaning out of everyday life,” he explains. “This drives them to take higher and higher doses of the opioid to maintain a dwindling sense of well-being.”
The changes are not just psychological: Chronic opioid use actually causes changes in the brain’s reward circuitry. “You can see on brain imaging,” says Garland. “The brain’s reward circuitry becomes more responsive when a person is viewing opioid related images than when they are viewing images of smiling babies, lovers holding hands, or sunsets over the beach.” MORE, he says, teaches “savoring” – a tenet of positive psychology – as a means of restructuring the reward processes in the brain so the patient becomes sensitive to pleasure from natural, healthy rewards, decreasing cravings for drug-related rewards.
Mindfulness and Addiction
Mindfulness, a form of meditation that teaches people to observe their feelings and sensations without judgement, has been increasingly applied to the treatment of addiction. By observing their pain and cravings objectively, for example, patients gain increased awareness of their responses to pain and their habits of opioid use. “They learn how to be with discomfort, whether emotional or physical, in a more compassionate way,” says Sarah Bowen, PhD, associate professor of psychology at Pacific University in Oregon. “And if your mind gives you a message like ‘Oh, I can’t handle that,’ to recognize that that’s a thought that might not be true.”
Bowen’s research is focused on Mindfulness-Based Relapse Prevention, which addresses the cravings associated with addiction. She has patients practice what she calls “urge surfing”: riding out a craving or urge rather than relying on a substance for immediate relief. “Craving will happen, so rather than fighting it, we look at understanding it better,” she says.
MORE differs from other forms of mindfulness-based therapy in that it integrates reappraisal and savoring training. Reappraisal is a technique often used in CBT in which patients learn to change negative thought patterns in order to reduce their emotional impact, while savoring helps to restructure the reward processes in the brain.
Mindfulness training not only helps patients to understand and gain control over their behavior in response to cravings and triggers like pain, says Garland, but also provides a means of pain relief. “We use mindfulness to zoom into pain and break it down into its subcomponents – feelings of heat or tightness or tingling – which reduces the impact that negative emotions have on pain processing in the brain.”

Eric Garland examines brain waves.
University of Utah
Powerful interventions
As the dangers of opioid addiction have become increasingly evident, some scientists are developing less addictive, non-opioid painkillers, but more trials are needed. Meanwhile, behavioral approaches to chronic pain relief have continued to gain traction, and researchers like Garland are probing the possibilities of integrative treatments to treat the addiction itself. Given that the number of people suffering from chronic pain and OUD have reached new heights during the COVID-19 pandemic, says Wachholtz, new treatment alternatives for patients caught in the relentless cycle of chronic pain and opioid misuse are sorely needed. “We’re trying to refine the techniques,” she says, “but we’re starting to realize just how powerful some of these mind-body interventions can be.”

