A New Stem Cell Therapy Provides Hope to Patients with Blood Cancer
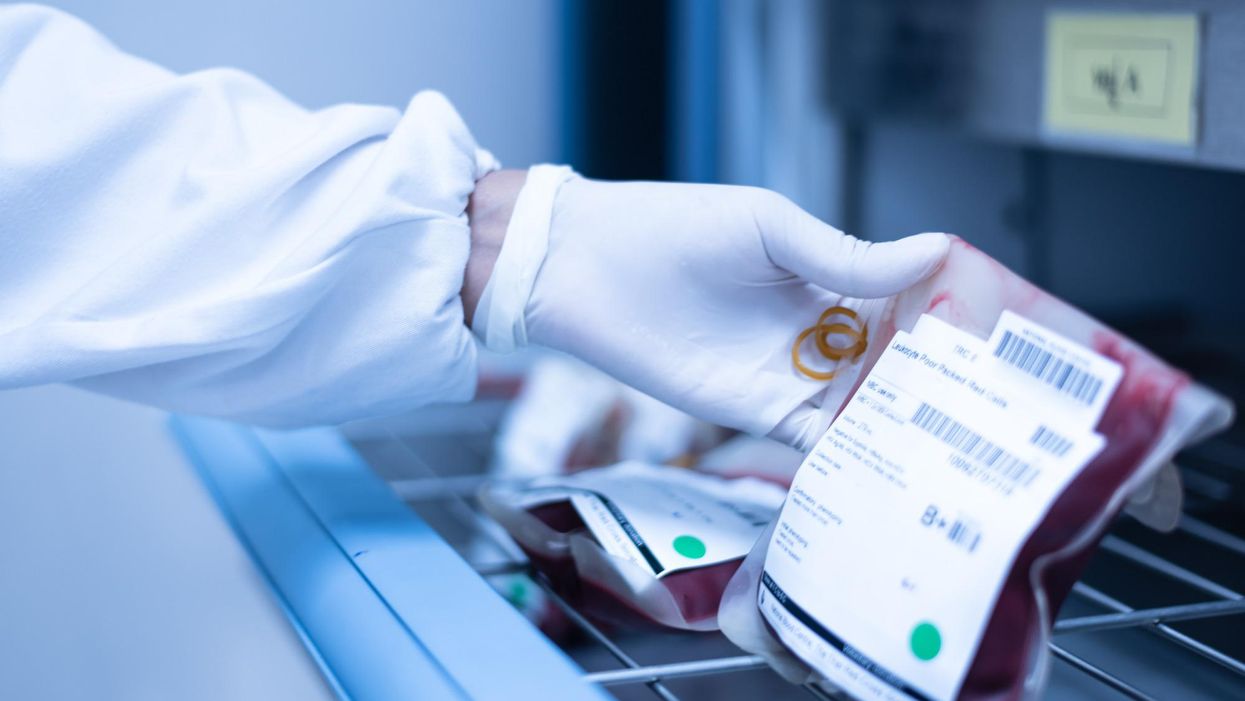
Stacey Khoury felt more fatigued and out of breath than she was used to from just walking up the steps to her job in retail jewelry sales in Nashville, Tennessee. By the time she got home, she was more exhausted than usual, too.
"I just thought I was working too hard and needed more exercise," recalls the native Nashvillian about those days in December 2010. "All of the usual excuses you make when you're not feeling 100%."
As a professional gemologist, being hospitalized during peak holiday sales season wasn't particularly convenient. There was no way around it though when her primary care physician advised Khoury to see a blood disorder oncologist because of her disturbing blood count numbers. As part of a routine medical exam, a complete blood count screens for a variety of diseases and conditions that affect blood cells, such as anemia, infection, inflammation, bleeding disorders and cancer.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before."
While she was in the hospital, a bone marrow biopsy revealed that Khoury had acute myeloid leukemia, or AML, a high-risk blood cancer. After Khoury completed an intense first round of chemotherapy, her oncologist recommended a bone marrow transplant. The potentially curative treatment for blood-cancer patients requires them to first receive a high dose of chemotherapy. Next, an infusion of stem cells from a healthy donor's bone marrow helps form new blood cells to fight off the cancer long-term.
Each year, approximately 8,000 patients in the U.S. with AML and other blood cancers receive a bone marrow transplant from a donor, according to the Center for International Blood and Marrow Transplant Research. But Khoury wasn't so lucky. She ended up being among the estimated 40% of patients eligible for bone marrow transplants who don't receive one, usually because there's no matched donor available.
Khoury's oncologist told her about another option. She could enter a clinical trial for an investigational cell therapy called omidubicel, which is being developed by Israeli biotech company Gamida Cell. The company's cell therapy, which is still experimental, could up a new avenue of treatment for cancer patients who can't get a bone marrow transplant.
Omidubicel consists of stem cells from cord blood that have been expanded using Gamida's technology to ensure there are enough cells for a therapeutic dose. The company's technology allows the immature cord blood cells to multiply quickly in the lab. Like a bone marrow transplant, the goal of the therapy is to make sure the donor cells make their way to the bone marrow and begin producing healthy new cells — a process called engraftment.
"If approved, it will allow more patients to potentially receive a transplant than would have gotten one before, so there's something very novel and exciting about that," says Ronit Simantov, Gamida Cell's chief medical officer.
Khoury and her husband Rick packed up their car and headed to the closest trial site, the Duke University School of Medicine, roughly 500 miles away. There they met with Mitchell Horowitz, a stem cell transplant specialist at Duke and principal investigator for Gamida's omidubicel study in the U.S.
He told Khoury she was a perfect candidate for the trial, and she enrolled immediately. "When you have one of two decisions, and it's either do this or you're probably not going to be around, it was a pretty easy decision to make, and I am truly thankful for that," she says.
Khoury's treatment started at the end of March 2011, and she was home by July 4 that year. She say the therapy "worked the way the doctors wanted it to work." Khoury's blood counts were rising quicker than the people who had bone marrow matches, and she was discharged from Duke earlier than other patients were.
By expanding the number of cord blood cells — which are typically too few to treat an adult — omidubicel allows doctors to use cord blood for patients who require a transplant but don't have a donor match for bone marrow.
Patients receiving omidubicel first get a blood test to determine their human leukocyte antigen, or HLA, type. This protein is found on most cells in the body and is an important regulator of the immune system. HLA typing is used to match patients to bone marrow and cord blood donors, but cord blood doesn't require as close of a match.
Like bone marrow transplants, one potential complication of omidubicel is graft-versus-host disease, when the donated bone marrow or stem cells register the recipient's body as foreign and attack the body. Depending on the severity of the response, according to the Mayo Clinic, treatment includes medication to suppress the immune system, such as steroids. In clinical trials, the occurrence of graft-versus-host disease with omidubicel was comparable with traditional bone marrow transplants.
"Transplant doctors are working on improving that," Simantov says. "A number of new therapies that specifically address graft-versus-host disease will be making some headway in the coming months and years."
Gamida released the results of the Phase 3 study in February and continues to follow Khoury and the other study patients for their long-term outcomes. The large randomized trial evaluated the safety and efficacy of omidubicel compared to standard umbilical cord blood transplants in patients with blood cancer who didn't have a suitable bone marrow donor. Around 120 patients aged 12 to 65 across the U.S., Europe and Asia were included in the trial. The study found that omidubicel resulted in faster recovery, fewer bacterial and viral infections and fewer days in the hospital.
The company plans to seek FDA approval this year. Simantov anticipates the therapy will receive FDA approval by 2022.
"Opening up cord blood transplants is very important, especially for people of diverse ethnic backgrounds," says oncologist Gary Schiller, principal investigator at the David Geffen School of Medicine at UCLA for Gamida Cell's mid- and late-stage trials. "This expansion technology makes a big difference because it makes cord blood an available option for those who do not have another donor source."
As for Khoury, who proudly celebrated the anniversary of her first transplant in April—she remains cancer free and continues to work full-time as a gemologist. When she has a little free time, she enjoys gardening, sewing, or maybe traveling to national parks like Yellowstone or the Grand Canyon with her husband Rick.
Although Jonas Salk has gone down in history for helping rid the world (almost) of polio, his revolutionary vaccine wouldn't have been possible without the world’s largest clinical trial – and the bravery of thousands of kids.
Exactly 67 years ago, in 1955, a group of scientists and reporters gathered at the University of Michigan and waited with bated breath for Dr. Thomas Francis Jr., director of the school’s Poliomyelitis Vaccine Evaluation Center, to approach the podium. The group had gathered to hear the news that seemingly everyone in the country had been anticipating for the past two years – whether the vaccine for poliomyelitis, developed by Francis’s former student Jonas Salk, was effective in preventing the disease.
Polio, at that point, had become a household name. As the highly contagious virus swept through the United States, cities closed their schools, movie theaters, swimming pools, and even churches to stop the spread. For most, polio presented as a mild illness, and was usually completely asymptomatic – but for an unlucky few, the virus took hold of the central nervous system and caused permanent paralysis of muscles in the legs, arms, and even people’s diaphragms, rendering the person unable to walk and breathe. It wasn’t uncommon to hear reports of people – mostly children – who fell sick with a flu-like virus and then, just days later, were relegated to spend the rest of their lives in an iron lung.
For two years, researchers had been testing a vaccine that would hopefully be able to stop the spread of the virus and prevent the 45,000 infections each year that were keeping the nation in a chokehold. At the podium, Francis greeted the crowd and then proceeded to change the course of human history: The vaccine, he reported, was “safe, effective, and potent.” Widespread vaccination could begin in just a few weeks. The nightmare was over.
The road to success
Jonas Salk, a medical researcher and virologist who developed the vaccine with his own research team, would rightfully go down in history as the man who eradicated polio. (Today, wild poliovirus circulates in just two countries, Afghanistan and Pakistan – with only 140 cases reported in 2020.) But many people today forget that the widespread vaccination campaign that effectively ended wild polio across the globe would have never been possible without the human clinical trials that preceded it.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. The consequences of polio had arguably never been more visible.
The road to human clinical trials – and the resulting vaccine – was a long one. In 1938, President Franklin Delano Roosevelt launched the National Foundation for Infantile Paralysis in order to raise funding for research and development of a polio vaccine. (Today, we know this organization as the March of Dimes.) A polio survivor himself, Roosevelt elevated awareness and prevention into the national spotlight, even more so than it had been previously. Raising funds for a safe and effective polio vaccine became a cornerstone of his presidency – and the funds raked in by his foundation went primarily to Salk to fund his research.
The Trials Begin
Salk’s vaccine, which included an inactivated (killed) polio virus, was promising – but now the researchers needed test subjects to make global vaccination a possibility. Because the aim of the vaccine was to prevent paralytic polio, researchers decided that they had to test the vaccine in the population that was most vulnerable to paralysis – young children. And, because the rate of paralysis was so low even among children, the team required many children to collect enough data. Francis, who led the trial to evaluate Salk’s vaccine, began the process of recruiting more than one million school-aged children between the ages of six and nine in 272 counties that had the highest incidence of the disease. The participants were nicknamed the “Polio Pioneers.”
Double-blind, placebo-based trials were considered the “gold standard” of epidemiological research back in Francis's day - and they remain the best approach we have today. These rigorous scientific studies are designed with two participant groups in mind. One group, called the test group, receives the experimental treatment (such as a vaccine); the other group, called the control, receives an inactive treatment known as a placebo. The researchers then compare the effects of the active treatment against the effects of the placebo, and every researcher is “blinded” as to which participants receive what treatment. That way, the results aren’t tainted by any possible biases.
But the study was controversial in that only some of the individual field trials at the county and state levels had a placebo group. Researchers described this as a “calculated risk,” meaning that while there were risks involved in giving the vaccine to a large number of children, the bigger risk was the potential paralysis or death that could come with being infected by polio. In all, just 200,000 children across the US received a placebo treatment, while an additional 725,000 children acted as observational controls – in other words, researchers monitored them for signs of infection, but did not give them any treatment.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. President Roosevelt, who had made many public and televised appearances in a wheelchair, served as a perpetual reminder of the consequences of polio, as an infection at age 39 had rendered him permanently unable to walk. The consequences of polio had arguably never been more visible, and parents signed up their children in droves to participate in the study and offer them protection.
The Polio Pioneer Legacy
In a little less than a year, roughly half a million children received a dose of Salk’s polio vaccine. While plenty of children were hesitant to get the shot, many former participants still remember the fear surrounding the disease. One former participant, a Polio Pioneer named Debbie LaCrosse, writes of her experience: “There was no discussion, no listing of pros and cons. No amount of concern over possible side effects or other unknowns associated with a new vaccine could compare to the terrifying threat of polio.” For their participation, each kid received a certificate – and sometimes a pin – with the words “Polio Pioneer” emblazoned across the front.
When Francis announced the results of the trial on April 12, 1955, people did more than just breathe a sigh of relief – they openly celebrated, ringing church bells and flooding into the streets to embrace. Salk, who had become the face of the vaccine at that point, was instantly hailed as a national hero – and teachers around the country had their students to write him ‘thank you’ notes for his years of diligent work.
But while Salk went on to win national acclaim – even accepting the Presidential Medal of Freedom for his work on the polio vaccine in 1977 – his success was due in no small part to the children (and their parents) who took a risk in order to advance medical science. And that risk paid off: By the early 1960s, the yearly cases of polio in the United States had gone down to just 910. Where before the vaccine polio had caused around 15,000 cases of paralysis each year, only ten cases of paralysis were recorded in the entire country throughout the 1970s. And in 1979, the virus that once shuttered entire towns was declared officially eradicated in this country. Thanks to the efforts of these brave pioneers, the nation – along with the majority of the world – remains free of polio even today.
Why you should (virtually) care
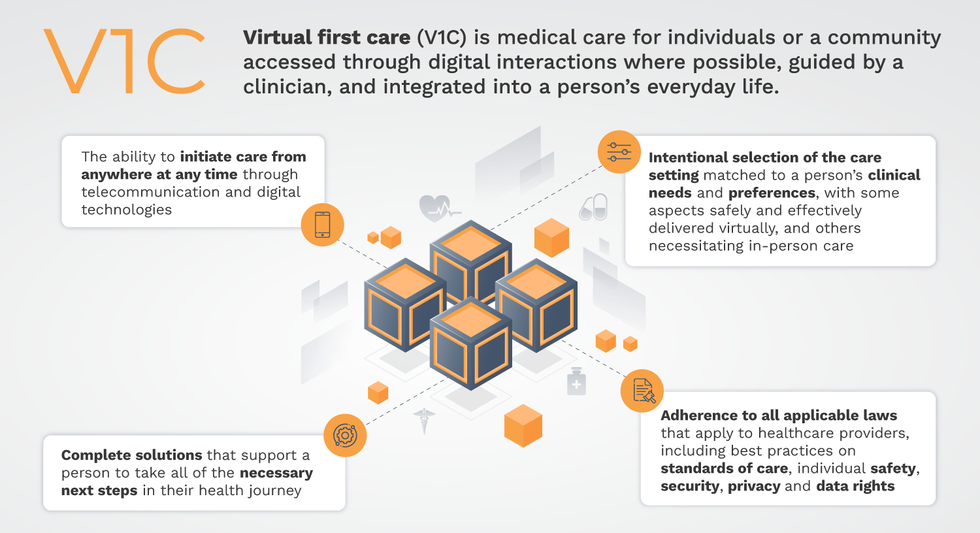
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.

