The Brave New World of Using DNA to Store Data

A panoramic view of DNA
Netscape co-founder-turned-venture capitalist billionaire investor Marc Andreessen once posited that software was eating the world. He was right, and the takeover of software resulted in many things. One of them is data. Lots and lots and lots of data. In the previous two years, humanity created more data than it did during its entire existence combined, and the amount will only increase. Think about it: The hundreds of 50KB emails you write a day, the dozens of 10MB photos, the minute-long, 350MB 4K video you shoot on your iPhone X add up to vast quantities of information. All that information needs to be stored. And that's becoming an issue as data volume outpaces storage space.
The race is on to find another medium capable of storing massive amounts of information in as small a space as possible.
"There won't be enough silicon to store all the data we need. It's unlikely that we can make flash memory smaller. We have reached the physical limits," Victor Zhirnov, chief scientist at the Semiconductor Research Corporation, says. "We are facing a crisis that's comparable to the oil crisis in the 1970s. By 2050, we're going to need to store 10 to the 30 bits, compared to 10 to the 23 bits in 2016." That amount of storage space is equivalent to each of the world's seven billion people owning almost six trillion -- that's 10 to the 12th power -- iPhone Xs with 256GB storage space.
The race is on to find another medium capable of storing massive amounts of information in as small a space as possible. Zhirnov and other scientists are looking at the human body, looking to DNA. "Nature has nailed it," Luis Ceze, a professor in the Department of Computer Science and Engineering at the University of Washington, says. "DNA is a molecular storage medium that is remarkable. It's incredibly dense, many, many thousands of times denser than the densest technology that we have today. And DNA is remarkably general. Any information you can map in bits you can store in DNA." It's so dense -- able to store a theoretical maximum of 215 petabytes (215 million gigabytes) in a single gram -- that all the data ever produced could be stored in the back of a tractor trailer truck.
Writing DNA can be an energy-efficient process, too. Consider how the human body is constantly writing and rewriting DNA, and does so on a couple thousand calories a day. And all it needs for storage is a cool, dark place, a significant energy savings when compared to server farms that require huge amounts of energy to run and even more energy to cool.
Picture it: tiny specks of inert DNA made from silicon or another material, stored in cool, dark, dry areas, preserved for all time.
Researchers first succeeded in encoding data onto DNA in 2012, when Harvard University geneticists George Church and Sri Kosuri wrote a 52,000-word book on A, C, G, and T base pairs. Their method only produced 1.28 petabytes per gram of DNA, however, a volume exceeded the next year when a group encoded all 154 Shakespeare sonnets and a 26-second clip of Martin Luther King's "I Have A Dream" speech. In 2017, Columbia University researchers Yaniv Erlich and Dina Zielinski made the process 60 percent more efficient.
The limiting factor today is cost. Erlich said the work his team did cost $7,000 to encode and decode two megabytes of data. To become useful in a widespread way, the price per megabyte needs to plummet. Even advocates concede this point. "Of course it is expensive," Zhirnov says. "But look how much magnetic storage cost in the 1980s. What you store today in your iPhone for virtually nothing would cost many millions of dollars in 1982." There's reason to think the price will continue to fall. Genome readers are improving, getting cheaper, faster, and smaller, and genome sequencing becomes cheaper every year, too. Picture it: tiny specks of inert DNA made from silicon or another material, stored in cool, dark, dry areas, preserved for all time.
"It just takes a few minutes to double a sample. A few more minutes, you double it again. Very quickly, you have thousands or millions of new copies."
Plus, DNA has another advantage over more traditional forms of storage: It's very easy to reproduce. "If you want a second copy of a hard disk drive, you need components for a disk drive, hook both drives up to a computer, and copy. That's a pain," Nick Goldman, a researcher at the European Bioinformatics Institute, says. "DNA, once you have that first sample, it's a process that is absolutely routine in thousands of laboratories around the world to multiply that using polymerase chain reaction [which uses temperature changes or other processes]. It just takes a few minutes to double a sample. A few more minutes, you double it again. Very quickly, you have thousands or millions of new copies."
This ability to duplicate quickly and easily is a positive trait. But, of course, there's also the potential for danger. Does encoding on DNA, the very basis for life, present ethical issues? Could it get out of control and fundamentally alter life as we know it?
The chance is there, but it's remote. The first reason is that storage could be done with only two base pairs, which would serve as replacements for the 0 and 1 digits that make up all digital data. While doing so would decrease the possible density of the storage, it would virtually eliminate the risk that the sequences would be compatible with life.
But even if scientists and researchers choose to use four base pairs, other safeguards are in place that will prevent trouble. According to Ceze, the computer science professor, the snippets of DNA that they write are very short, around 150 nucleotides. This includes the title, the information that's being encoded, and tags to help organize where the snippet should fall in the larger sequence. Furthermore, they generally avoid repeated letters, which dramatically reduces the chance that a protein could be synthesized from the snippet.
"In the future, we'll know enough about someone from a sample of their DNA that we could make a specific poison. That's the danger, not those of us who want to encode DNA for storage."
Inevitably, some DNA will get spilt. "But it's so unlikely that anything that gets created for storage would have a biological interpretation that could interfere with the mechanisms going on in a living organism that it doesn't worry me in the slightest," Goldman says. "We're not of concern for the people who are worried about the ethical issues of synthetic DNA. They are much more concerned about people deliberately engineering anthrax. In the future, we'll know enough about someone from a sample of their DNA that we could make a specific poison. That's the danger, not those of us who want to encode DNA for storage."
In the end, the reality of and risks surrounding encoding on DNA are the same as any scientific advancement: It's another system that is vulnerable to people with bad intentions but not one that is inherently unethical.
"Every human action has some ethical implications," Zhirnov says. "I can use a hammer to build a house or I can use it to harm another person. I don't see why DNA is in any way more or less ethical."
If that house can store all the knowledge in human history, it's worth learning how to build it.
Editor's Note: In response to readers' comments that silicon is one of the earth's most abundant materials, we reached back out to our source, Dr. Victor Zhirnov. He stands by his statement about a coming shortage of silicon, citing this research. The silicon oxide found in beach sand is unsuitable for semiconductors, he says, because the cost of purifying it would be prohibitive. For use in circuit-making, silicon must be refined to a purity of 99.9999999 percent. So the process begins by mining for pure quartz, which can only be found in relatively few places around the world.
Scientists have known about and studied heart rate variability, or HRV, for a long time and, in recent years, monitors have come to market that can measure HRV accurately.
This episode is about a health metric you may not have heard of before: heart rate variability, or HRV. This refers to the small changes in the length of time between each of your heart beats.
Scientists have known about and studied HRV for a long time. In recent years, though, new monitors have come to market that can measure HRV accurately whenever you want.
Five months ago, I got interested in HRV as a more scientific approach to finding the lifestyle changes that work best for me as an individual. It's at the convergence of some important trends in health right now, such as health tech, precision health and the holistic approach in systems biology, which recognizes how interactions among different parts of the body are key to health.
But HRV is just one of many numbers worth paying attention to. For this episode of Making Sense of Science, I spoke with psychologist Dr. Leah Lagos; Dr. Jessilyn Dunn, assistant professor in biomedical engineering at Duke; and Jason Moore, the CEO of Spren and an app called Elite HRV. We talked about what HRV is, research on its benefits, how to measure it, whether it can be used to make improvements in health, and what researchers still need to learn about HRV.
*Talk to your doctor before trying anything discussed in this episode related to HRV and lifestyle changes to raise it.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Show notes
Spren - https://www.spren.com/
Elite HRV - https://elitehrv.com/
Jason Moore's Twitter - https://twitter.com/jasonmooreme?lang=en
Dr. Jessilyn Dunn's Twitter - https://twitter.com/drjessilyn?lang=en
Dr. Dunn's study on HRV, flu and common cold - https://jamanetwork.com/journals/jamanetworkopen/f...
Dr. Leah Lagos - https://drleahlagos.com/
Dr. Lagos on Star Talk - https://www.youtube.com/watch?v=jC2Q10SonV8
Research on HRV and intermittent fasting - https://pubmed.ncbi.nlm.nih.gov/33859841/
Research on HRV and Mediterranean diet - https://medicalxpress.com/news/2010-06-twin-medite...:~:text=Using%20data%20from%20the%20Emory,eating%20a%20Western%2Dtype%20diet
Devices for HRV biofeedback - https://elitehrv.com/heart-variability-monitors-an...
Benefits of HRV biofeedback - https://pubmed.ncbi.nlm.nih.gov/32385728/
HRV and cognitive performance - https://www.frontiersin.org/articles/10.3389/fnins...
HRV and emotional regulation - https://pubmed.ncbi.nlm.nih.gov/36030986/
Fortune article on HRV - https://fortune.com/well/2022/12/26/heart-rate-var...
Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
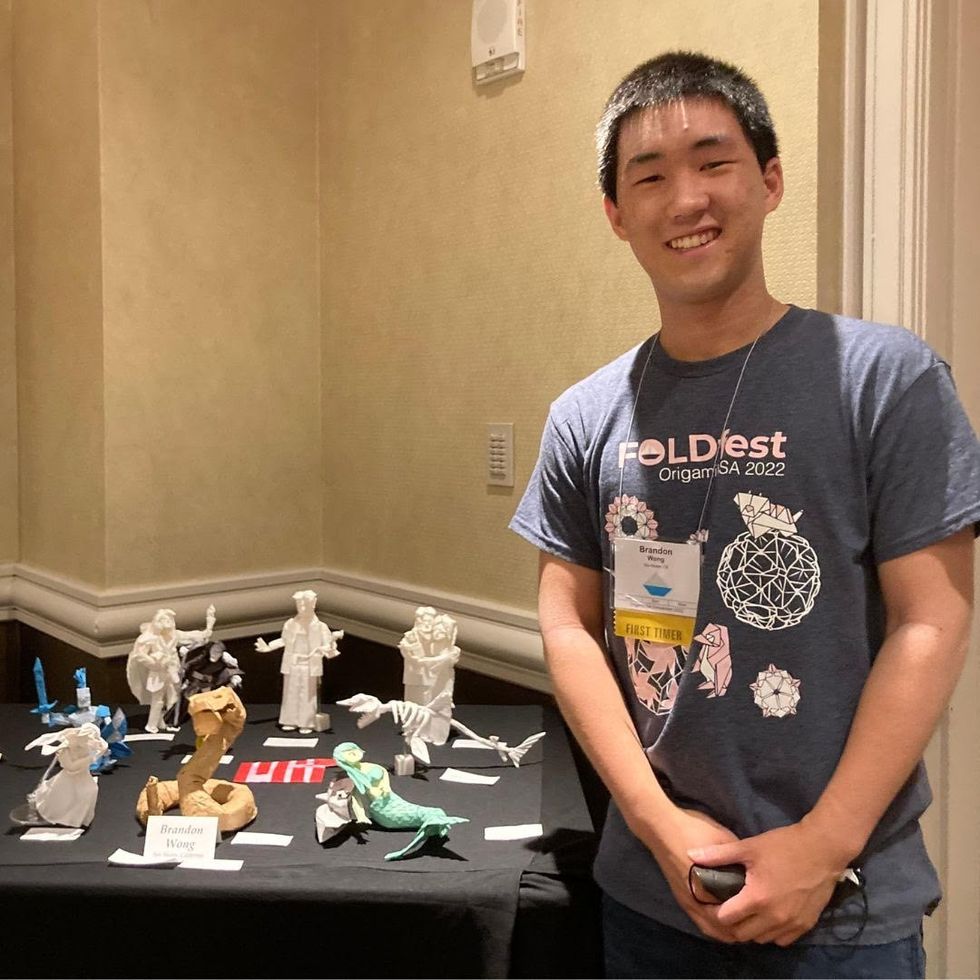
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”

