Can Cultured Meat Save the Planet?
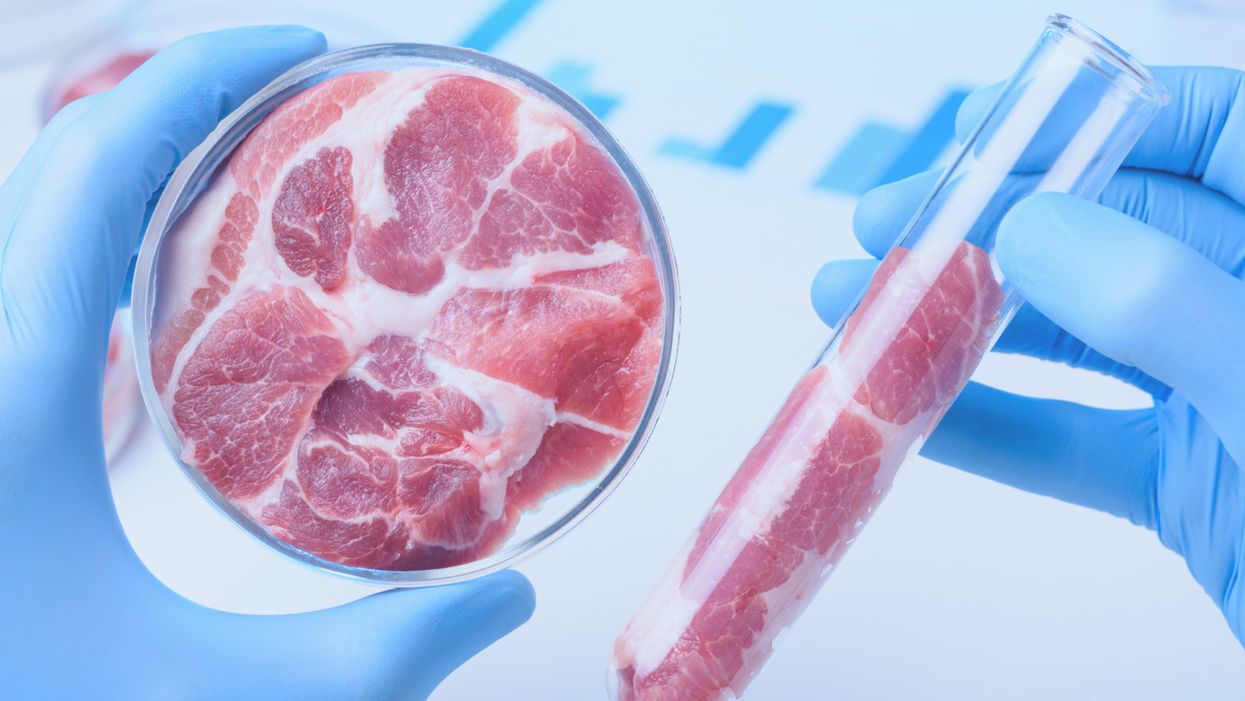
Lab-grown meat in a Petri dish and test tube.
In September, California governor Jerry Brown signed a bill mandating that by 2045, all of California's electricity will come from clean power sources. Technological breakthroughs in producing electricity from sun and wind, as well as lowering the cost of battery storage, have played a major role in persuading Californian legislators that this goal is realistic.
Even if the world were to move to an entirely clean power supply, one major source of greenhouse gas emissions would continue to grow: meat.
James Robo, the CEO of the Fortune 200 company NextEra Energy, has predicted that by the early 2020s, electricity from solar farms and giant wind turbines will be cheaper than the operating costs of coal-fired power plants, even when the cost of storage is included.
Can we therefore all breathe a sigh of relief, because technology will save us from catastrophic climate change? Not yet. Even if the world were to move to an entirely clean power supply, and use that clean power to charge up an all-electric fleet of cars, buses and trucks, one major source of greenhouse gas emissions would continue to grow: meat.
The livestock industry now accounts for about 15 percent of global greenhouse gas emissions, roughly the same as the emissions from the tailpipes of all the world's vehicles. But whereas vehicle emissions can be expected to decline as hybrids and electric vehicles proliferate, global meat consumption is forecast to be 76 percent greater in 2050 than it has been in recent years. Most of that growth will come from Asia, especially China, where increasing prosperity has led to an increasing demand for meat.
Changing Climate, Changing Diets, a report from the London-based Royal Institute of International Affairs, indicates the threat posed by meat production. At the UN climate change conference held in Cancun in 2010, the participating countries agreed that to allow global temperatures to rise more than 2°C above pre-industrial levels would be to run an unacceptable risk of catastrophe. Beyond that limit, feedback loops will take effect, causing still more warming. For example, the thawing Siberian permafrost will release large quantities of methane, causing yet more warming and releasing yet more methane. Methane is a greenhouse gas that, ton for ton, warms the planet 30 times as much as carbon dioxide.
The quantity of greenhouse gases we can put into the atmosphere between now and mid-century without heating up the planet beyond 2°C – known as the "carbon budget" -- is shrinking steadily. The growing demand for meat means, however, that emissions from the livestock industry will continue to rise, and will absorb an increasing share of this remaining carbon budget. This will, according to Changing Climate, Changing Diets, make it "extremely difficult" to limit the temperature rise to 2°C.
One reason why eating meat produces more greenhouse gases than getting the same food value from plants is that we use fossil fuels to grow grains and soybeans and feed them to animals. The animals use most of the energy in the plant food for themselves, moving, breathing, and keeping their bodies warm. That leaves only a small fraction for us to eat, and so we have to grow several times the quantity of grains and soybeans that we would need if we ate plant foods ourselves. The other important factor is the methane produced by ruminants – mainly cattle and sheep – as part of their digestive process. Surprisingly, that makes grass-fed beef even worse for our climate than beef from animals fattened in a feedlot. Cattle fed on grass put on weight more slowly than cattle fed on corn and soybeans, and therefore do burp and fart more methane, per kilogram of flesh they produce.
Richard Branson has suggested that in 30 years, we will look back on the present era and be shocked that we killed animals en masse for food.
If technology can give us clean power, can it also give us clean meat? That term is already in use, by advocates of growing meat at the cellular level. They use it, not to make the parallel with clean energy, but to emphasize that meat from live animals is dirty, because live animals shit. Bacteria from the animals' guts and shit often contaminates the meat. With meat cultured from cells grown in a bioreactor, there is no live animal, no shit, and no bacteria from a digestive system to get mixed into the meat. There is also no methane. Nor is there a living animal to keep warm, move around, or grow body parts that we do not eat. Hence producing meat in this way would be much more efficient, and much cleaner, in the environmental sense, than producing meat from animals.
There are now many startups working on bringing clean meat to market. Plant-based products that have the texture and taste of meat, like the "Impossible Burger" and the "Beyond Burger" are already available in restaurants and supermarkets. Clean hamburger meat, fish, dairy, and other animal products are all being produced without raising and slaughtering a living animal. The price is not yet competitive with animal products, but it is coming down rapidly. Just this week, leading officials from the Food and Drug Administration and the U.S. Department of Agriculture have been meeting to discuss how to regulate the expected production and sale of meat produced by this method.
When Kodak, which once dominated the sale and processing of photographic film, decided to treat digital photography as a threat rather than an opportunity, it signed its own death warrant. Tyson Foods and Cargill, two of the world's biggest meat producers, are not making the same mistake. They are investing in companies seeking to produce meat without raising animals. Justin Whitmore, Tyson's executive vice-president, said, "We don't want to be disrupted. We want to be part of the disruption."
That's a brave stance for a company that has made its fortune from raising and killing tens of billions of animals, but it is also an acknowledgement that when new technologies create products that people want, they cannot be resisted. Richard Branson, who has invested in the biotech company Memphis Meats, has suggested that in 30 years, we will look back on the present era and be shocked that we killed animals en masse for food. If that happens, technology will have made possible the greatest ethical step forward in the history of our species, saving the planet and eliminating the vast quantity of suffering that industrial farming is now inflicting on animals.
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

