Can Radical Transparency Overcome Resistance to COVID-19 Vaccines?

Secretive panels of independent experts called Data Safety and Monitoring Boards examine clinical trials' data for safety and efficacy.
When historians look back on the COVID-19 pandemic, they may mark November 9, 2020 as the day the tide began to turn. That's when the New York-based pharmaceutical giant Pfizer announced that clinical trials showed its experimental vaccine, developed with the German firm BioNTech, to be 90 percent effective in preventing the disease.
A week later, Massachusetts biotech startup Moderna declared its vaccine to be 95 percent effective. By early December, Great Britain had begun mass inoculations, followed—once the Food and Drug Administration gave the thumbs-up—by the United States. In this scenario, the worst global health crisis in a century was on the cusp of resolution.
Yet future chroniclers may instead peg November 9 as the day false hope dawned. That could happen if serious safety issues, undetected so far, arise after millions of doses are administered. Experts consider it unlikely, however, that such problems alone (as opposed to the panic they might spark) would affect enough people to thwart a victory over the coronavirus. A more immediate obstacle is vaccine hesitancy—the prospect that much of the populace will refuse to roll up their sleeves.
To achieve "herd immunity" for COVID-19 (the point at which a vaccine reduces transmission rates enough to protect those who can't or won't take it, or for whom it doesn't work), epidemiologists estimate that up to 85 percent of the population will have to be vaccinated. Alarmingly, polls suggest that 40 to 50 percent of Americans intend to decline, judging the risks to be more worrisome than those posed by the coronavirus itself.
COVID vaccine skeptics occupy various positions on a spectrum of doubt. Some are committed anti-vaxxers, or devotees of conspiracy theories that view the pandemic as a hoax. Others belong to minority groups that have historically been used as guinea pigs in unethical medical research (for horrific examples, Google "Tuskegee syphilis experiment" or "Henrietta Lacks"). Still others simply mistrust Big Pharma and/or Big Government. A common fear is that the scramble to find a vaccine—intensified by partisan and profit motives—has led to corner-cutting in the testing and approval process. "They really rushed," an Iowa trucker told The Washington Post. "I'll probably wait a couple of months after they start to see how everyone else is handling it."
The COVID crisis has spurred calls for secretive Data Safety and Monitoring Boards to come out of the shadows.
The consensus among scientists, by contrast, is that the process has been rigorous enough, given the exigency of the situation, that the public can feel reasonably confident in any vaccine that has earned the imprimatur of the FDA. For those of us who share that assessment, finding ways to reassure the hesitant-but-persuadable is an urgent matter.
Vax-positive public health messaging is one obvious tactic, but a growing number of experts say it's not enough. They prescribe a regimen of radical transparency throughout the system that regulates research—in particular, regarding the secretive panels that oversee vaccine trials.
The Crucial Role of the Little-Known Panels
Like other large clinical trials involving potentially high-demand or controversial products, studies of COVID-19 vaccines in most countries are supervised by groups of independent observers. Known in the United States as data safety and monitoring boards (DSMBs), and elsewhere as data monitoring committees, these panels consist of scientists, clinicians, statisticians, and other authorities with no ties to the sponsor of the study.
The six trials funded by the federal program known as Operation Warp Speed (including those of newly approved Moderna and frontrunner AstraZeneca) share a DSMB, whose members are selected by the National Institutes of Health; other companies (including Pfizer) appoint their own. The panel's job is to monitor the safety and efficacy of a treatment while the trial is ongoing, and to ensure that data is being collected and analyzed correctly.
Vaccine studies are "double-blinded," which means neither the participants nor the doctors running the trial know who's getting the real thing and who's getting a placebo. But the DSMB can access that information if a study volunteer has what might be a serious side effect—and if the participant was in the vaccine group, the board can ask that the trial be paused for further investigation.
The DSMB also checks for efficacy at pre-determined intervals. If it finds that the vaccine group and the placebo group are getting sick at similar rates, the panel can recommend stopping the trial due to "futility." And if the results look overwhelmingly positive, the DSMB can recommend that the study sponsor apply for FDA approval before the scheduled end of the trial, in order to hurry the product to market.
With this kind of inside dope and high-level influence, DSMBs could easily become targets for outside pressure. That's why, since the 1980s, their membership has typically been kept secret.
During the early days of the AIDS crisis, researchers working on HIV drugs feared for the safety of the experts on their boards. "They didn't want them to be besieged and harassed by members of the community," explains Susan Ellenberg, a professor of biostatistics, medical ethics and health policy at the University of Pennsylvania, and co-author of Data Monitoring Committees in Clinical Trials, the DSMB bible. "You can understand why people would very much want to know how things were looking in a given trial. They wanted to save their own lives; they wanted to save their friends' lives." Ellenberg, who was founding director of the biostatistics branch of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID), helped shape a range of policies designed to ensure that DSMBs made decisions based on data and nothing else.
Confidentiality also shields DSMB members from badgering by patient advocacy groups, who might urge that a drug be presented for approval before trial results are conclusive, or by profit-hungry investors. "It prevents people from trying to pry out information to get an edge in the stock market," says Art Caplan, a bioethicist at New York University.
Yet the COVID crisis has spurred calls for DSMBs to come out of the shadows. One triggering event came in March 2020, when the FDA approved hydroxychloroquine for COVID-19—a therapy that President Donald J. Trump touted, despite scant evidence for its efficacy. (Approval was rescinded in June.) If the agency could bow to political pressure on these medications, critics warned, it might do so with vaccines as well. In the end, that didn't happen; the Pfizer approval was issued well after Election Day, despite Trump's goading, and most experts agree that it was based on solid science. Still, public suspicion lingers.
Another shock came in September, after British-based AstraZeneca announced it was pausing its vaccine trial globally due to a "suspected adverse rection" in a volunteer. The company shared no details with the press. Instead, AstraZeneca's CEO divulged them in a private call with J.P. Morgan investors the next day, confirming that the volunteer was suffering from transverse myelitis, a rare and serious spinal inflammation—and that the study had also been halted in July, when another volunteer displayed neurological symptoms. STAT News broke the story after talking to tipsters.
Although both illnesses were found to be unrelated to the vaccine, and the trial was restarted, the incident had a paradoxical effect: while it confirmed for experts that the oversight system was working, AstraZeneca's initial lack of candor added to many laypeople's sense that it wasn't. "If you were seeking to undermine trust, that's kind of how you would go about doing it," says Charles Weijer, a bioethicist at Western University in Ontario, who has helped develop clinical trial guidelines for the World Health Organization.
Both Caplan and Weijer have served on many DSMBs; they believe the boards are generally trustworthy, and that those overseeing COVID vaccine trials are performing their jobs well. But the secrecy surrounding these groups, they and others argue, has become counterproductive. Shining a light on the statistical sausage-makers would help dispel doubts about the finished product.
"I'm not suggesting that any of these companies are doing things unethically," Weijer explains. "But the circumstances of a global pandemic are sufficiently challenging that perhaps they ought to be doing some things differently. I believe it would be trust-producing for data monitoring committees to be more forthcoming than usual."
Building Trust: More Transparency
Just how forthcoming is a matter of debate. Caplan suggests that each COVID vaccine DSMB reveal the name of its chair; that would enable the scientific community, as well as the media and the general public, to get a sense of the integrity and qualifications of the board as a whole while preserving the anonymity of the other members.
Indeed, when Operation Warp Speed's DSMB chair, Richard Whitley, was outed through a website slip-up, many observers applauded his selection for the role; a professor of pediatrics, microbiology, medicine and neurosurgery at the University of Alabama at Birmingham, he is "an exceptionally experienced and qualified individual," Weijer says. (Reporters with ProPublica later identified two other members: Susan Ellenberg and immunologist William Makgoba, known for his work on the South African AIDS Vaccine Initiative.)
Caplan would also like to see more details of the protocols DSMBs are using to make decisions, such as the statistical threshold for efficacy that would lead them to seek approval from the FDA. And he wishes the NIH would spell out specific responsibilities for these monitoring boards. "They don't really have clear, government-mandated charters," he notes. For example, there's no requirement that DSMBs include an ethicist or patient advocate—both of which Caplan considers essential for vaccine trials. "Rough guidelines," he says, "would be useful."
Weijer, for his part, thinks DSMBs should disclose all their members. "When you only disclose the chair, you leave questions unanswered," he says. "What expertise do [the others] bring to the table? Are they similarly free of relevant conflicts of interest? And it doesn't answer the question that will be foremost on many people's minds: are these people in the pocket of pharma?"
Weijer and Caplan both want to see greater transparency around the trial results themselves. Because the FDA approved the Pfizer and Moderna vaccines with emergency use authorizations rather than full licensure, which requires more extensive safety testing, these products reached the market without the usual paper trail of peer-reviewed publications. The same will likely be true of any future COVID vaccines that the agency greenlights. To add another level of scrutiny, both ethicists suggest, each company should publicly release its data at the end of a trial. "That offers the potential for academic groups to go in and do an analysis," Weijer explains, "to verify the claims about the safety and efficacy of the vaccine." The point, he says, is not only to ensure that the approval was justified, but to provide evidence to counter skeptics' qualms.
Caplan may differ on some of the details, but he endorses the premise. "It's all a matter of trust," he says. "You're always watching that, because a vaccine is only as good as the number of people who take it."
The Friday Five: How to exercise for cancer prevention
How to exercise for cancer prevention. Plus, a device that brings relief to back pain, ingredients for reducing Alzheimer's risk, the world's oldest disease could make you young again, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- How to exercise for cancer prevention
- A device that brings relief to back pain
- Ingredients for reducing Alzheimer's risk
- Is the world's oldest disease the fountain of youth?
- Scared of crossing bridges? Your phone can help
New approach to brain health is sparking memories
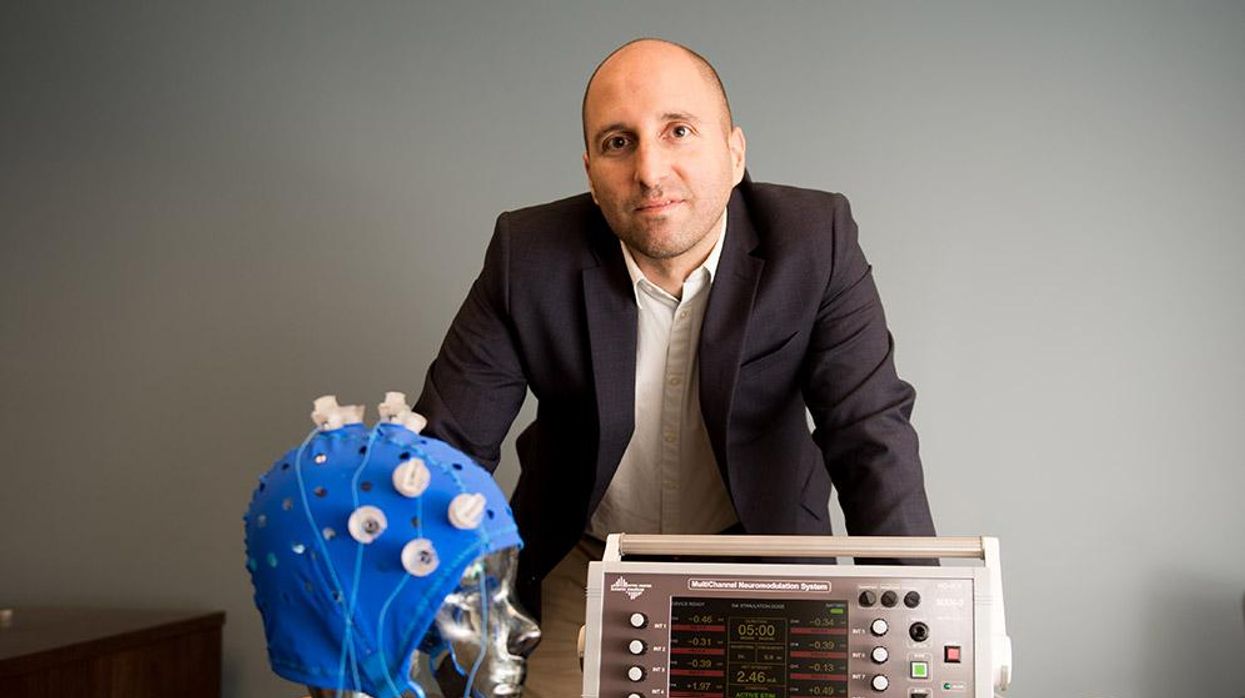
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”