Can Spare Parts from Pigs Solve Our Organ Shortage?
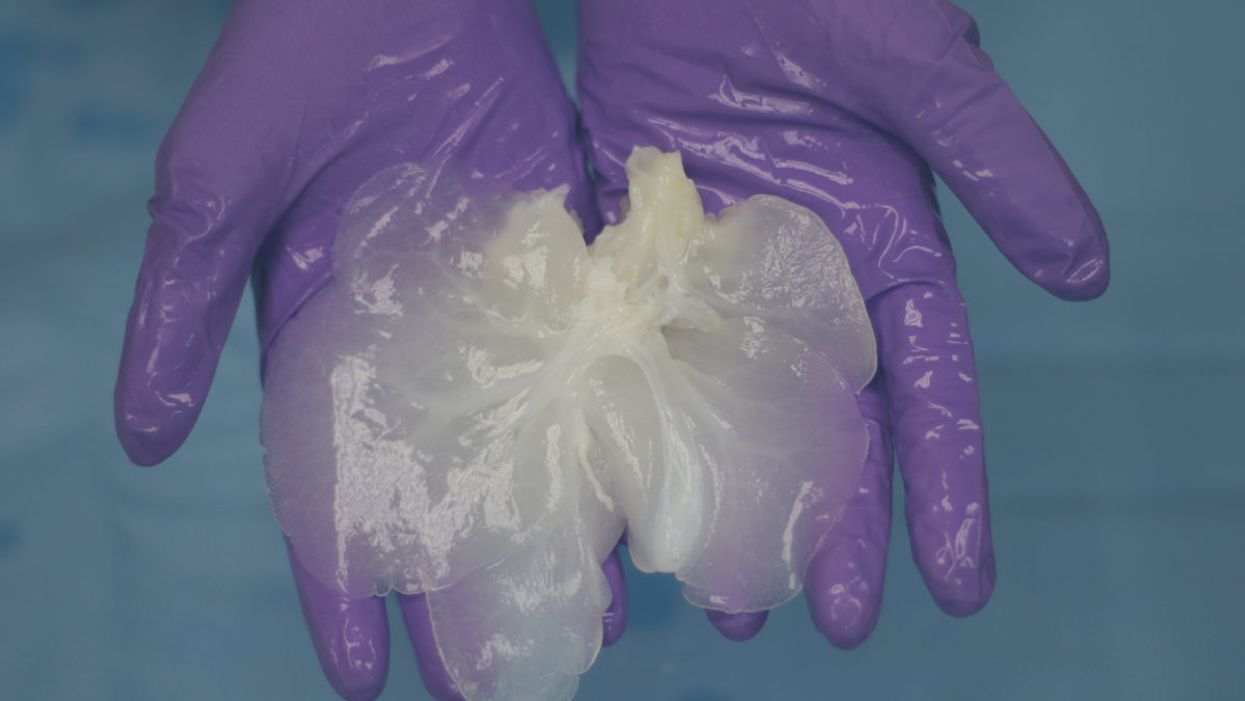
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."
Opioid prescription policies may hurt those in chronic pain
Guidelines aim to prevent opioid-related deaths by making it more challenging to get prescriptions, but they can also block access for those who desperately need them.
Tinu Abayomi-Paul works as a writer and activist, plus one unwanted job: Trying to fill her opioid prescription. She says that some pharmacists laugh and tell her that no one needs the amount of pain medication that she is seeking. Another pharmacist near her home in Venus, Tex., refused to fill more than seven days of a 30-day prescription.
To get a new prescription—partially filled opioid prescriptions can’t be dispensed later—Abayomi-Paul needed to return to her doctor’s office. But without her medication, she was having too much pain to travel there, much less return to the pharmacy. She rationed out the pills over several weeks, an agonizing compromise that left her unable to work, interact with her children, sleep restfully, or leave the house. “Don’t I deserve to do more than survive?” she says.
Abayomi-Paul’s pain results from a degenerative spine disorder, chronic lymphocytic leukemia, and more than a dozen other diagnoses and disabilities. She is part of a growing group of people with chronic pain who have been negatively impacted by the fallout from efforts to prevent opioid overdose deaths.
Guidelines for dispensing these pills are complicated because many opioids, like codeine, oxycodone, and morphine, are prescribed legally for pain. Yet, deaths from opioids have increased rapidly since 1999 and become a national emergency. Many of them, such as heroin, are used illegally. The CDC identified three surges in opioid use: an increase in opioid prescriptions in the ‘90s, a surge of heroin around 2010, and an influx of fentanyl and other powerful synthetic opioids in 2013.
As overdose deaths grew, so did public calls to address them, prompting the CDC to change its prescription guidelines in 2016. The new guidelines suggested limiting medication for acute pain to a seven-day supply, capping daily doses of morphine, and other restrictions. Some statistics suggest that these policies have worked; from 2016 to 2019, prescriptions for opiates fell 44 percent. Physicians also started progressively lowering opioid doses for patients, a practice called tapering. A study tracking nearly 100,000 Medicare subscribers on opioids found that about 13 percent of patients were tapering in 2012, and that number increased to about 23 percent by 2017.
But some physicians may be too aggressive with this tapering strategy. About one in four people had doses reduced by more than 10 percent per week, a rate faster than the CDC recommends. The approach left people like Abayomi-Paul without the medication they needed. Every year, Abayomi-Paul says, her prescriptions are harder to fill. David Brushwood, a pharmacy professor who specializes in policy and outcomes at the University of Florida in Gainesville, says opioid dosing isn’t one-size-fits-all. “Patients need to be taken care of individually, not based on what some government agency says they need,” he says.
‘This is not survivable’
Health policy and disability rights attorney Erin Gilmer advocated for people with pain, using her own experience with chronic pain and a host of medical conditions as a guidepost. She launched an advocacy website, Healthcare as a Human Right, and shared her struggles on Twitter: “This pain is more than anything I've endured before and I've already been through too much. Yet because it's not simply identified no one believes it's as bad as it is. This is not survivable.”
When her pain dramatically worsened midway through 2021, Gilmer’s posts grew ominous: “I keep thinking it can't possibly get worse but somehow every day is worse than the last.”
The CDC revised its guidelines in 2022 after criticisms that people with chronic pain were being undertreated, enduring dangerous withdrawal symptoms, and suffering psychological distress. (Long-term opioid use can cause physical dependency, an adaptive reaction that is different than the compulsive misuse associated with a substance use disorder.) It was too late for Gilmer. On July 7, 2021, the 38-year-old died by suicide.
Last August, an Ohio district court ruling set forth a new requirement for Walgreens, Walmart, and CVS pharmacists in two counties. These pharmacists must now document opioid prescriptions that are turned down, even for customers who have no previous purchases at that pharmacy, and they’re required to share this information with other locations in the same chain. None of the three pharmacies responded to an interview request from Leaps.org.
In a practice called red flagging, pharmacists may label a prescription suspicious for a variety of reasons, such as if a pharmacist observes an unusually high dose, a long distance from the patient’s home to the pharmacy, or cash payment. Pharmacists may question patients or prescribers to resolve red flags but, regardless of the explanation, they’re free to refuse to fill a prescription.
As the risk of litigation has grown, so has finger-pointing, says Seth Whitelaw, a compliance consultant at Whitelaw Compliance Group in West Chester, PA, who advises drug, medical device, and biotech companies. Drugmakers accused in National Prescription Opioid Litigation (NPOL), a complex set of thousands of cases on opioid epidemic deaths, which includes the Ohio district case, have argued that they shouldn’t be responsible for the large supply of opiates and overdose deaths. Yet, prosecutors alleged that these pharmaceutical companies hid addiction and overdose risks when labeling opioids, while distributors and pharmacists failed to identify suspicious orders or scripts.
Patients and pharmacists fear red flags
The requirements that pharmacists document prescriptions they refuse to fill so far only apply to two counties in Ohio. But Brushwood fears they will spread because of this precedent, and because there’s no way for pharmacists to predict what new legislation is on the way. “There is no definition of a red flag, there are no lists of red flags. There is no instruction on what to do when a red flag is detected. There’s no guidance on how to document red flags. It is a standardless responsibility,” Brushwood says. This adds trepidation for pharmacists—and more hoops to jump through for patients.
“I went into the doctor one day here and she said, ‘I'm going to stop prescribing opioids to all my patients effective immediately,” Nicolson says.
“We now have about a dozen studies that show that actually ripping somebody off their medication increases their risk of overdose and suicide by three to five times, destabilizes their health and mental health, often requires some hospitalization or emergency care, and can cause heart attacks,” says Kate Nicolson, founder of the National Pain Advocacy Center based in Boulder, Colorado. “It can kill people.” Nicolson was in pain for decades due to a surgical injury to the nerves leading to her spinal cord before surgeries fixed the problem.
Another issue is that primary care offices may view opioid use as a reason to turn down new patients. In a 2021 study, secret shoppers called primary care clinics in nine states, identifying themselves as long-term opioid users. When callers said their opioids were discontinued because their former physician retired, as opposed to an unspecified reason, they were more likely to be offered an appointment. Even so, more than 40 percent were refused an appointment. The study authors say their findings suggest that some physicians may try to avoid treating people who use opioids.
Abayomi-Paul says red flagging has changed how she fills prescriptions. “Once I go to one place, I try to [continue] going to that same place because of the amount of records that I have and making sure my medications don’t conflict,” Abayomi-Paul says.
Nicolson moved to Colorado from Washington D.C. in 2015, before the CDC issued its 2016 guidelines. When the guidelines came out, she found the change to be shockingly abrupt. “I went into the doctor one day here and she said, ‘I'm going to stop prescribing opioids to all my patients effective immediately.’” Since then, she’s spoken with dozens of patients who have been red-flagged or simply haven’t been able to access pain medication.
Despite her expertise, Nicolson isn’t positive she could successfully fill an opioid prescription today even if she needed one. At this point, she’s not sure exactly what various pharmacies would view as a red flag. And she’s not confident that these red flags even work. “You can have very legitimate reasons for being 50 miles away or having to go to multiple pharmacies, given that there are drug shortages now, as well as someone refusing to fill [a prescription.] It doesn't mean that you’re necessarily ‘drug seeking.’”
While there’s no easy solution. Whitelaw says clarifying the role of pharmacists and physicians in patient access to opioids could help people get the medication they need. He is seeking policy changes that focus on the needs of people in pain more than the number of prescriptions filled. He also advocates standardizing the definition of red flags and procedures for resolving them. Still, there will never be a single policy that can be applied to all people, explains Brushwood, the University of Florida professor. “You have to make a decision about each individual prescription.”
Recent immigration restrictions have left many foreign researchers' projects and careers in limbo—and some in jeopardy.
This article is part of the magazine, "The Future of Science In America: The Election Issue," co-published by LeapsMag, the Aspen Institute Science & Society Program, and GOOD.
When COVID-19 cases were surging in New York City in early spring, Chitra Mohan, a postdoctoral fellow at Weill Cornell, was overwhelmed with worry. But the pandemic was only part of her anxieties. Having come to the United States from India on a student visa that allowed her to work for a year after completing her degree, she had applied for a two-year extension, typically granted for those in STEM fields. But due to a clerical error—Mohan used an electronic signatureinstead of a handwritten one— her application was denied and she could no longerwork in the United States.
"I was put on unpaid leave and I lost my apartment and my health insurance—and that was in the middle of COVID!" she says.
Meanwhile her skills were very much needed in those unprecedented times. A molecular biologist studying how DNA can repair itself, Mohan was trained in reverse transcription polymerase chain reaction or RT-PCR—a lab technique that detects pathogens and is used to diagnose COVID-19. Mohan wanted to volunteer at testing centers, but because she couldn't legally work in the U.S., she wasn't allowed to help either. She moved to her cousin's house, hired a lawyer, and tried to restore her work status.
"I spent about $4,000 on lawyer fees and another $1,200 to pay for the motions I filed," she recalls. "I had to borrow money from my parents and my cousin because without my salary I just didn't have the $7,000 at hand." But the already narrow window of opportunity slammed completely shut when the Trump administration suspended issuing new visas for foreign researchers in June. All Mohan's attempts were denied. In August, she had to leave the country. "Given the recent work visa ban by the administration, all my options in the U.S. are closed," she wrote a bitter note on Twitter. "I have to uproot my entire life in NY for the past 6 years and leave." She eventually found a temporary position in Calcutta, where she can continue research.
Mohan is hardly alone in her visa saga. Many foreign scholars on H- and J-type visas and other permits that let them remain employed in America had been struggling to keep their rights to continue research, which in certain cases is crucial to battling the pandemic. Some had to leave the country, some filed every possible extension to buy time, and others are stuck in their home countries, unable to return. The already cumbersome process of applying for visas and extensions became crippled during the lockdowns. But in June, when President Trump extended and expanded immigration restrictions to cut the number of immigrant workers entering the U.S., the new limits left researchers' projects and careers in limbo—and some in jeopardy.
"We have been a beneficiary of this flow of human capacity and resource investment for many generations—and this is now threatened."
Rakesh Ramachandran, whose computational biology work contributed to one of the first coronavirus studies to map out its protein structures—is stranded in India. In early March, he had travelled there to attend a conference and visit the American consulate to stamp his H1 visa for a renewal, already granted. The pandemic shut down both the conference and the consulates, and Ramachandran hasn't been able to come back since. The consulates finally opened in September, but so far the online portal has no available appointment slots. "I'm told to keep trying," Ramachandran says.
The visa restrictions affected researchers worldwide, regardless of disciplines or countries. A Ph.D. student in neuroscience, Morgane Leroux had to do her experiments with mice at Gladstone Institutes in America and analyze the data back home at Sorbonne University in France. She had finished her first round of experiments when the lockdowns forced her to return to Paris, and she hasn't been able to come back to resume her work since. "I can't continue the experiments, which is really frustrating," she says, especially because she doesn't know what it means for her Ph.D. "I may have to entirely change my subject," she says, which she doesn't want to do—it would be a waste of time and money.
But besides wreaking havoc in scholars' personal lives and careers, the visa restrictions had—and will continue to have—tremendous deleterious effects on America's research and its global scientific competitiveness. "It's incredibly short-sighted and self-destructing to restrict the immigration of scientists into the U.S.," says Benjamin G. Neel, who directs the Laura and Isaac Perlmutter Cancer Center at New York University. "If they can't come here, they will go elsewhere," he says, causing a brain drain.

Neel in his lab with postdocs
(Courtesy of Neel)
Neel felt the outcomes of the shortsighted policies firsthand. In the past few months, his lab lost two postdoctoral researchers who had made major strides in understanding the biology of several particularly stubborn, treatment-resistant malignancies. One postdoc studied the underlying mechanisms responsible for 90 percent of pancreatic cancers and half of the colon ones. The other one devised a new system of modeling ovarian cancer in mice to test new therapeutic drug combinations for the deadliest tumor types—but had to return home to China.
"By working around the clock, she was able to get her paper accepted, but she hasn't been able to train us to use this new system, which can set us back six months," Neel says.
Her discoveries also helped the lab secure about $900,000 in grants for new research. Losing people like this is "literally killing the goose that lays the golden eggs," Neel adds. "If you want to make America poor again, this is the way to do it."
Cassidy R. Sugimoto at Indiana University Bloomington, who studies how scientific knowledge is produced and disseminated, says that scientists are the most productive when they are free to move, exchange ideas, and work at labs with the best equipment. Restricting that freedom reduces their achievement.
"Several empirical studied demonstrated the benefits to the U.S. by attracting and retaining foreign scientists. The disproportional number of our Nobel Prize winners were not only foreign-born but also foreign-educated," she says. Scientific advancement bolsters the country's economic prowess, too, so turning scholars away is bad for the economy long-term. "We have been a beneficiary of this flow of human capacity and resource investment for many generations—and this is now threatened," Sugimoto adds—because scientists will look elsewhere. "We are seeing them shifting to other countries that are more hospitable, both ideologically and in terms of health security. Many visiting scholars, postdocs, and graduate students who would otherwise come to the United States are now moving to Canada."
It's not only the Ph.D. students and postdocs who are affected. In some cases, even well-established professors who have already made their marks in the field and direct their own labs at prestigious research institutions may have to pack up and leave the country in the next few months. One scientist who directs a prominent neuroscience lab is betting on his visa renewal and a green card application, but if that's denied, the entire lab may be in jeopardy, as many grants hinge on his ability to stay employed in America.
"It's devastating to even think that it can happen," he says—after years of efforts invested. "I can't even comprehend how it would feel. It would be terrifying and really sad." (He asked to withhold his name for fear that it may adversely affect his applications.) Another scientist who originally shared her story for this article, later changed her mind and withdrew, worrying that speaking out may hurt the entire project, a high-profile COVID-19 effort. It's not how things should work in a democratic country, scientists admit, but that's the reality.
Still, some foreign scholars are speaking up. Mehmet Doğan, a physicist at University of California Berkeley who has been fighting a visa extension battle all year, says it's important to push back in an organized fashion with petitions and engage legislators. "This administration was very creative in finding subtle and not so subtle ways to make our lives more difficult," Doğan says. He adds that the newest rules, proposed by the Department of Homeland Security on September 24, could further limit the time scholars can stay, forcing them into continuous extension battles. That's why the upcoming election might be a turning point for foreign academics. "This election will decide if many of us will see the U.S. as the place to stay and work or whether we look at other countries," Doğan says, echoing the worries of Neel, Sugimoto, and others in academia.

Dogan on Zoom talking to his fellow union members of the Academic Researchers United, a union of almost 5,000 Academic Researchers.
(Credit: Ceyda Durmaz Dogan)
If this year has shown us anything, it is that viruses and pandemics know no borders as they sweep across the globe. Likewise, science can't be restrained by borders either. "Science is an international endeavor," says Neel—and right now humankind now needs unified scientific research more than ever, unhindered by immigration hurdles and visa wars. Humanity's wellbeing in America and beyond depends on it.
[Editor's Note: To read other articles in this special magazine issue, visit the beautifully designed e-reader version.]
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

