Can Spare Parts from Pigs Solve Our Organ Shortage?
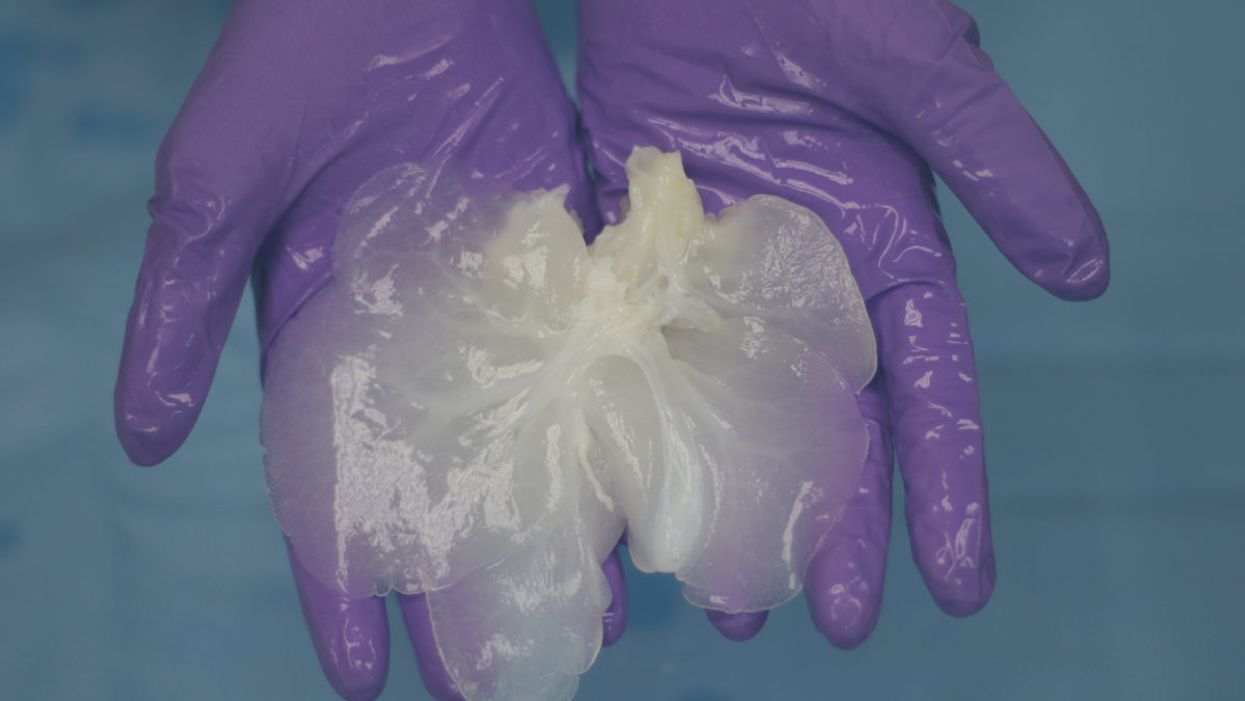
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."
Story by Big Think
We live in strange times, when the technology we depend on the most is also that which we fear the most. We celebrate cutting-edge achievements even as we recoil in fear at how they could be used to hurt us. From genetic engineering and AI to nuclear technology and nanobots, the list of awe-inspiring, fast-developing technologies is long.
However, this fear of the machine is not as new as it may seem. Technology has a longstanding alliance with power and the state. The dark side of human history can be told as a series of wars whose victors are often those with the most advanced technology. (There are exceptions, of course.) Science, and its technological offspring, follows the money.
This fear of the machine seems to be misplaced. The machine has no intent: only its maker does. The fear of the machine is, in essence, the fear we have of each other — of what we are capable of doing to one another.
How AI changes things
Sure, you would reply, but AI changes everything. With artificial intelligence, the machine itself will develop some sort of autonomy, however ill-defined. It will have a will of its own. And this will, if it reflects anything that seems human, will not be benevolent. With AI, the claim goes, the machine will somehow know what it must do to get rid of us. It will threaten us as a species.
Well, this fear is also not new. Mary Shelley wrote Frankenstein in 1818 to warn us of what science could do if it served the wrong calling. In the case of her novel, Dr. Frankenstein’s call was to win the battle against death — to reverse the course of nature. Granted, any cure of an illness interferes with the normal workings of nature, yet we are justly proud of having developed cures for our ailments, prolonging life and increasing its quality. Science can achieve nothing more noble. What messes things up is when the pursuit of good is confused with that of power. In this distorted scale, the more powerful the better. The ultimate goal is to be as powerful as gods — masters of time, of life and death.
Should countries create a World Mind Organization that controls the technologies that develop AI?
Back to AI, there is no doubt the technology will help us tremendously. We will have better medical diagnostics, better traffic control, better bridge designs, and better pedagogical animations to teach in the classroom and virtually. But we will also have better winnings in the stock market, better war strategies, and better soldiers and remote ways of killing. This grants real power to those who control the best technologies. It increases the take of the winners of wars — those fought with weapons, and those fought with money.
A story as old as civilization
The question is how to move forward. This is where things get interesting and complicated. We hear over and over again that there is an urgent need for safeguards, for controls and legislation to deal with the AI revolution. Great. But if these machines are essentially functioning in a semi-black box of self-teaching neural nets, how exactly are we going to make safeguards that are sure to remain effective? How are we to ensure that the AI, with its unlimited ability to gather data, will not come up with new ways to bypass our safeguards, the same way that people break into safes?
The second question is that of global control. As I wrote before, overseeing new technology is complex. Should countries create a World Mind Organization that controls the technologies that develop AI? If so, how do we organize this planet-wide governing board? Who should be a part of its governing structure? What mechanisms will ensure that governments and private companies do not secretly break the rules, especially when to do so would put the most advanced weapons in the hands of the rule breakers? They will need those, after all, if other actors break the rules as well.
As before, the countries with the best scientists and engineers will have a great advantage. A new international détente will emerge in the molds of the nuclear détente of the Cold War. Again, we will fear destructive technology falling into the wrong hands. This can happen easily. AI machines will not need to be built at an industrial scale, as nuclear capabilities were, and AI-based terrorism will be a force to reckon with.
So here we are, afraid of our own technology all over again.
What is missing from this picture? It continues to illustrate the same destructive pattern of greed and power that has defined so much of our civilization. The failure it shows is moral, and only we can change it. We define civilization by the accumulation of wealth, and this worldview is killing us. The project of civilization we invented has become self-cannibalizing. As long as we do not see this, and we keep on following the same route we have trodden for the past 10,000 years, it will be very hard to legislate the technology to come and to ensure such legislation is followed. Unless, of course, AI helps us become better humans, perhaps by teaching us how stupid we have been for so long. This sounds far-fetched, given who this AI will be serving. But one can always hope.
Interview with Jamie Metzl: We need a global OS upgrade
Jamie Metzl, author of Hacking Darwin, shares his views with Leaps.org on the future of genetics, tech, healthcare and more.
In this Q&A, leading technology and healthcare futurist Jamie Metzl discusses a range of topics and trend lines that will unfold over the next several decades: whether a version of Moore's Law applies to genetic technologies, the ethics of genetic engineering, the dangers of gene hacking, the end of sex, and much more.
Metzl is a member of the WHO expert advisory committee on human genome editing and the bestselling author of Hacking Darwin.
The conversation was lightly edited by Leaps.org for style and length.
In Hacking Darwin, you describe how we may modify the human body with CRISPR technologies, initially to obtain unsurpassed sports performance and then to enhance other human characteristics. What would such power over human biology mean for the future of our civilization?
After nearly four billion years of evolution, our one species suddenly has the increasing ability to read, write, and hack the code of life. This will have massive implications across the board, including in human health and reproduction, plant and animal agriculture, energy and advanced materials, and data storage and computing, just to name a few. My book Hacking Darwin: Genetic Engineering and the Future of Humanity primarly explored how we are currently deploying and will increasingly use our capabilities to transform human life in novel ways. My next book, The Great Biohack: Recasting Life in an Age of Revolutionary Technology, coming out in May 2024, will examine the broader implications for all of life on Earth.
We humans will, over time, use these technologies on ourselves to solve problems and eventually to enhance our capabilities. We need to be extremely conservative, cautious, and careful in doing so, but doing so will almost certainly be part of our future as a species.

In electronics, Moore's law is an established theory that computing power doubles every 18 months. Is there any parallel to be drawn with genetic technologies?
The increase in speed and decrease in costs of genome sequencing have progressed far faster than Moore’s law. It took thirteen years and cost about a billion dollars to sequence the first human genome. Today it takes just a few hours and can cost as little as a hundred dollars to do a far better job. In 2012, Jennifer Doudna and Emmanuel Charpentier published the basic science paper outlining the CRISPR-cas9 genome editing tool that would eventually win them the Nobel prize. Only six years later, the first CRISPR babies were born in China. If it feels like technology is moving ever-faster, that’s because it is.
Let's turn to the topic of aging. Do you think that the field of genetics will advance fast enough to eventually increase maximal lifespan for a child born this year? How about for a person who is currently age 50?
The science of aging is definitely real, but that doesn’t mean we will live forever. Aging is a biological process subject to human manipulation. Decades of animal research shows that. This does not mean we will live forever, but it does me we will be able to do more to expand our healthspans, the period of our lives where we are able to live most vigorously.
The first thing we need to do is make sure everyone on earth has access to the resources necessary to live up to their potential. I live in New York City, and I can take a ten minute subway ride to a neighborhood where the average lifespan is over a decade shorter than in mine. This is true within societies and between countries as well. Secondly, we all can live more like people in the Blue Zones, parts of the world where people live longer, on average, than the rest of us. They get regular exercise, eat healthy foods, have strong social connections, etc. Finally, we will all benefit, over time, from more scientific interventions to extend our healthspan. This may include small molecule drugs like metformin, rapamycin, and NAD+ boosters, blood serum infusions, and many other things.
Science fiction has depicted a future where we will never get sick again, stay young longer or become immortal. Assuming that any of this is remotely possible, should we be afraid of such changes, even if they seem positive in some regards, because we can’t understand the full implications at this point?
Not all of these promises will be realized in full, but we will use these technologies to help us live healthier, longer lives. We will never become immortal becasue nothing lasts forever. We will always get sick, even if the balance of diseases we face shifts over time, as it has always done. It is healthy, and absolutely necessary, that we feel both hope and fear about this future. If we only feel hope, we will blind ourselves to the very real potential downsides. If we only feel fear, we will deny ourselves the very meaningful benefits these technologies have the potential to provide.
A fascinating chapter in Hacking Darwin is entitled The End of Sex. And you see that as a good thing?
We humans will always be a sexually reproducing species, it’s just that we’ll reproduce increasingly less through the physical act of sex. We’re already seeing this with IVF. As the benefits of technology assisted reproduction increase relative to reproduction through the act of sex, many people will come to see assisted reproduction as a better way to reduce risk and, over time, possibly increase benefits. We’ll still have sex for all the other wonderful reasons we have it today, just less for reproduction. There will always be a critical place in our world for Italian romantics!
What are dangers of genetic hackers, perhaps especially if everyone’s DNA is eventually transcribed for medical purposes and available on the internet and in the cloud?
The sky is really the limit for how we can use gentic technologies to do things we may want, and the sky is also the limit for potential harms. It’s quite easy to imagine scenarios in which malevolent actors create synthetic pathogens designed to wreak havoc, or where people steal and abuse other people’s genetic information. It wouldn’t even need to be malevolent actors. Even well-intentioned researchers making unintended mistakes could cause real harm, as we may have seen with COVID-19 if, as appears likely to me, the pandemic stems for a research related incident]. That’s why we need strong governance and regulatory systems to optimize benefits and minimize potential harms. I was honored to have served on the World Health Organization Expert Advisory Committee on Human Genome Editing, were we developed a proposed framework for how this might best be achieved.
You foresee the equivalent of a genetic arms race between the world's most powerful countries. In what sense are genetic technologies similar to weapons?
Genetic technologies could be used to create incredibly powerful bioweapons or to build gene drives with the potential to crash entire ecosystems. That’s why thoughtful regulation is in order. Because the benefits of mastering and deploying these technologies are so great, there’s also a real danger of a genetics arms race. This could be extremely dangerous and will need to be prevented.
In your book, you express concern that states lacking Western conceptions of human rights are especially prone to misusing the science of genetics. Does this same concern apply to private companies? How much can we trust them to control and wield these technologies?
This is a conversation about science and technology but it’s really a conversation about values. If we don’t agree on what core values should be promoted, it will be nearly impossible to agree on what actions do and do not make sense. We need norms, laws, and values frameworks that apply to everyone, including governments, corporations, researchers, healthcare providers, DiY bio hobbyists, and everyone else.
We have co-evolved with our technology for a very long time. Many of our deepest beliefs have formed in that context and will continue to do so. But as we take for ourselves the powers we have attributed to our various gods, many of these beliefs will be challenged. We can not and must not jettison our beliefs in the face of technology, and must instead make sure our most cherished values guide the application of our most powerful technologies.
A conversation on international norms is in full swing in the field of AI, prompted by the release of ChatGPT4 earlier this year. Are there ways in which it’s inefficient, shortsighted or otherwise problematic for these discussions on gene technologies, AI and other advances to be occurring in silos? In addition to more specific guidelines, is there something to be gained from developing a universal set of norms and values that applies more broadly to all innovation?
AI is yet another technology where the potential to do great good is tied to the potential to inflict signifcant harm. It makes no sense that we tend to treat each technology on its own rather than looking at the entire category of challenges. For sure, we need to very rapidly ramp up our efforts with regard to AI norm-setting, regulations, and governance at all levels. But just doing that will be kind of like generating a flu vaccine for each individual flu strain. Far better to build a universal flu vaccine addressing common elements of all flu viruses of concern.
That’s why we also need to be far more deliberate in both building a global operating systems based around the mutual responsibilities of our global interdependence and, under that umbrella, a broader system for helping us govern and regulate revolutionary technologies. Such a process might begin with a large international conference, the equivalent of Rio 1992 for climate change, but then quickly work to establish and share best practices, help build parallel institutions in all countries so people and governamts can talk with each other, and do everything possible to maximize benefits and minimize risks at all levels in an ongoing and dynamic way.
At what point might genetic enhancements lead to a reclassfication of modified humans as another species?
We’ll still all be fellow humans for a very, very long time. We already have lots of variation between us. That is the essence of biology. Will some humans, at some point in the future, leave Earth and spend generations elsewhere? I believe so. In those new environments, humans will evolve, over time, differently than those if us who remain on this planet? This may sound like science fiction, but the sci-fi future is coming at us faster than most people realize.
Is the concept of human being changing?
Yes. It always has and always will.
Another big question raised in your book: what limits should we impose on the freedom to manipulate genetics?
Different societies will come to different conclusion on this critical question. I am sympathetic to the argument that people should have lots of say over their own bodies, which why I support abortion rights even though I recognize that an abortion can be a violent procedure. But it would be insane and self-defeating to say that individuals have an unlimited right to manipulate their own or their future children’s heritable genetics. The future of human life is all of our concern and must be regulated, albeit wisely.
In some cases, such as when we have the ability to prevent a deadly genetic disroder, it might be highly ethical to manipulate other human beings. In other circumstances, the genetic engineering of humans might be highly unethical. The key point is to avoid asking this question in a binary manner. We need to weigh the costs and benefits of each type of intervention. We need societal and global infrastrucutres to do that well. We don’t yet have those but we need them badly.
Can you tell us more about your next book?
The Great Biohack: Recasting Lifee in an Age of Revolutionary Technology, will come out in May 2024. It explores what the intersecting AI, genetics, and biotechnology revolutions will mean for the future of life on earth, including our healthcare, agriculture, industry, computing, and everything else. We are at a transitional moment for life on earth, equivalent to the dawn of agriculture, electricity, and industrialization. The key differentiator between better and worse outcomes is what we do today, at this early stage of this new transformation. The book describes what’s happening, what’s at stake, and what we each and all can and, frankly, must do to build the type of future we’d like to inhabit.
You’ve been a leader of international efforts calling for a full investigation into COVID-19 origins and are the founder of the global movement OneShared.World. What problem are you trying to solve through OneShared.World?
The biggest challenge we face today is the mismatch between the nature of our biggest problems, global and common, and the absence of a sufficient framework for addressing that entire category of challenges. The totally avoidable COVID-19 pandemic is one example of the extremet costs of the status quo. OneShared.World is our effort to fight for an upgrade in our world’s global operating system, based around the mutual responsibilities of interdependence. We’ve had global OS upgrades before after the Thirty Years War and after World War II, but wouldn’t it be better to make the necessary changes now to prevent a crisis of that level stemming from a nuclear war, ecosystem collapse, or deadlier synthetic biology pandemic rather than waiting until after? Revolutionary science is a global issue that must be wisely managed at every level if it is to be wisely managed at all.
How do we ensure that revolutionary technologies benefit humanity instead of undermining it?
That is the essential question. It’s why I’ve written Hacking Darwin, am writing The Great Biohack, and doing the rest of my work. If we want scietific revolutions to help, rather than hurt, us, we must all play a role building that future. This isn’t just a conversation about science, it’s about how we can draw on our most cherished values to guide the optimal development of science and technology for the common good. That must be everyone’s business.
Portions of this interview were first published in Grassia (Italy) and Zen Portugal.
Jamie Metzl is one of the world’s leading technology and healthcare futurists and author of the bestselling book, Hacking Darwin: Genetic Engineering and the Future of Humanity, which has been translated into 15 languages. In 2019, he was appointed to the World Health Organization expert advisory committee on human genome editing. Jamie is a faculty member of Singularity University and NextMed Health, a Senior Fellow of the Atlantic Council, and Founder and Chair of the global social movement, OneShared.World.
Called “the original COVID-19 whistleblower,” his pioneering role advocating for a full investigation into the origins of the COVID-19 pandemic has been featured in 60 Minutes, the New York Times, and most major media across the globe, and he was the lead witness in the first congressional hearings on this topic. Jamie previously served in the U.S. National Security Council, State Department, and Senate Foreign Relations Committee and with the United Nations in Cambodia. Jamie appears regularly on national and international media and his syndicated columns and other writing in science, technology, and global affairs are featured in publications around the world.
Jamie sits on advisory boards for multiple biotechnology and other companies and is Special Strategist to the WisdomTree BioRevolution Exchange Traded Fund. In addition to Hacking Darwin, he is author of a history of the Cambodian genocide, the historical novel The Depths of the Sea, and the genetics sci-fi thrillers Genesis Code and Eternal Sonata. His next book, The Great Biohack: Recasting Life in an age of Revolutionary Technology, will be published by Hachette in May 2024. Jamie holds a Ph.D. from Oxford, a law degree from Harvard, and an undergraduate degree from Brown and is an avid ironman triathlete and ultramarathon runner.


