Can Spare Parts from Pigs Solve Our Organ Shortage?
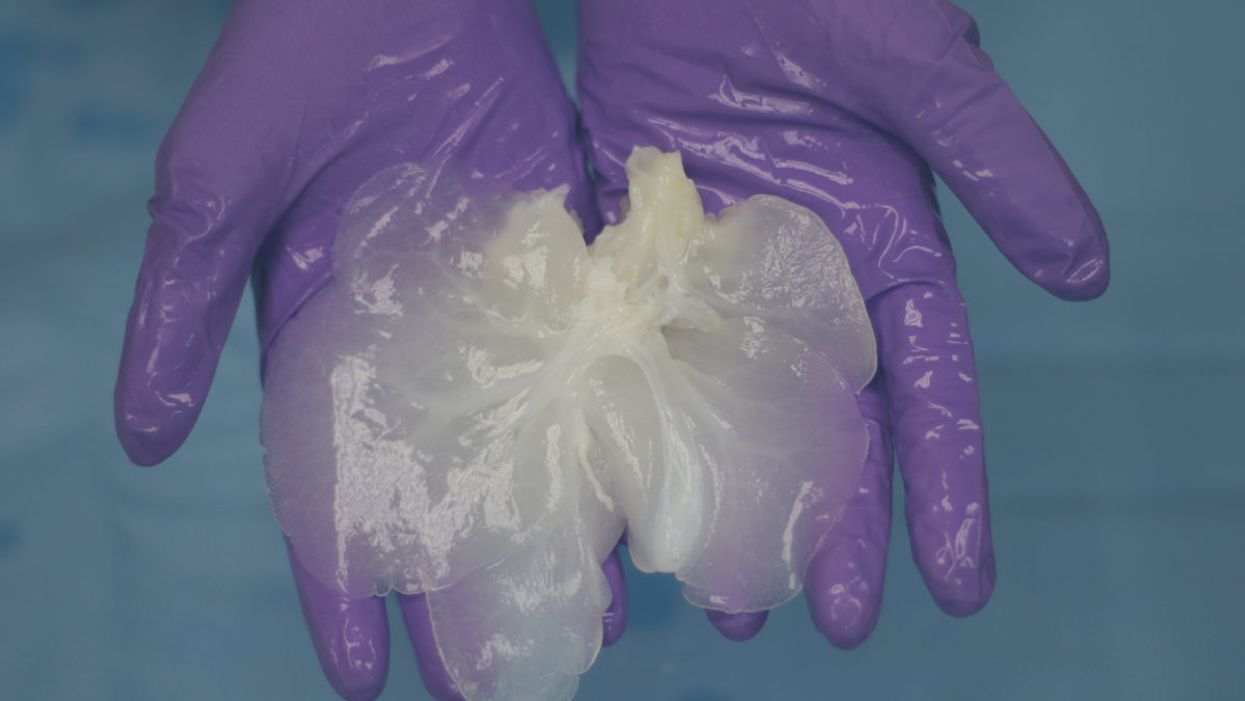
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."
Scientists aim to preserve donkeys, one frozen embryo at a time
In Ethiopia, Samuna’s three donkeys help her transport produce to market and to collect the water essential to her family, neighbours and livestock. Donkeys are more endangered than people realize, experts say.
Every day for a week in 2022, Andres Gambini, a veterinarian and senior lecturer in animal science at the University of Queensland in Australia, walked into his lab—and headed straight to the video camera. Trained on an array of about 50 donkey embryos, all created by Gambini’s manual in vitro fertilization, or IVF, the camera kept an eye on their developmental progress. To eventually create a viable embryo that could be implanted into a female donkey, the embryos’ cells had to keep dividing, first in two, then in four and so on.
But the embryos weren’t cooperating. Some would start splitting up only to stop a day or two later, and others wouldn’t start at all. Every day he came in, Gambini saw fewer and fewer dividing embryos, so he was losing faith in the effort. “You see many failed attempts and get disappointed,” he says.
Gambini and his team, a group of Argentinian and Spanish researchers, were working to create these embryos because many donkey populations around the world are declining. It may sound counterintuitive that domesticated animals may need preservation, but out of 28 European donkey breeds, 20 are endangered and seven are in critical status. It is partly because of the inbreeding that happened over the course of many years and partly because in today’s Western world donkeys aren’t really used anymore.
“That's the reason why some breeds begin to disappear because humans were not really interested in having that specific breed anymore,” Gambini says. Nonetheless, in Africa, India and Latin America millions of rural families still rely on these hardy creatures for agriculture and transportation. And the only two wild donkey species—Equus africanus in Africa and Equus hemionus in Asia—are also dwindling, due to losing their habitats to human activities, diseases and slow reproduction rates. Gambini’s team wanted to create a way to preserve the animals for the future. “Donkeys are more endangered than people realize,” he says.
There’s much more to donkeys' trouble though. For the past 20 or so years, they have been facing a huge existential threat due to their hide gelatin, a compound derived from their skins by soaking and stewing. In Chinese traditional medicine, the compound, called ejiao, is believed to have a medicinal value, so it’s used in skin creams, added to food and taken in capsules. Centuries ago, ejiao was a very expensive luxury product available only for the emperor and his household. That changed in the 1990s when the Chinese economy boomed, and many people were suddenly able to afford it. “It went from a very elite product to a very popular product,” says Janneke Merkx, a campaign manager at The Donkey Sanctuary, a United Kingdom-based nonprofit organization that keeps tabs on the animals’ welfare worldwide. “It is a status symbol for gift giving.”
Having evolved in the harsh and arid mountainous terrains where food and water were scarce, donkeys are extremely adaptable and hardy. But the Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement.
The Chinese donkey population was quickly decimated. Unlike many other farm animals, donkeys are finicky breeders. When stressed and unhappy, they don’t procreate, so growing them in large industrial settings isn’t possible. “Donkeys are notoriously slow breeders and really very difficult to farm,” says Merkx. “They are not the same as other livestock like sheep and pigs and cattle.” Within years the, the donkey numbers in China dropped precipitously. “China used to have the largest donkey population in the world in the 1990s. They had 11 million donkeys, and it's now down to less than 3 million, and they just can't keep up with the demand.”
To keep the ejiao conveyor going, some producers turned to the illegal wildlife trade. Poachers began to steal and slaughter donkeys from rural villages in Africa. The Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement. Exactly how many creatures were lost to the skin trade to-date isn’t possible to calculate, says Faith Burden, the Donkey Sanctuary’s director of equine operations. Traditionally a poor people’s beast of burden, donkey counts are hard to keep track of. “When an animal doesn't produce meat, milk or eggs or whatever edible product, they're often less likely to be acknowledged in a government population census,” Burden says. “So reliable statistics are hard to come by.” The nonprofit estimates that about 4.8 million are slaughtered annually.
During their six to seven thousand years of domestication, donkeys rarely got the full appreciation for their services. They are often compared to horses, which doesn’t do them justice. They’re entirely different animals, Burden says. Built for speed, horses respond to predators and other dangers by running as fast as they can. Donkeys, which originate from the rocky, mountainous regions of Africa where running is dangerous, react to threats by freezing and assessing the situation for the best response. “Those so-called stubborn donkeys that won’t move as you want, they are actually thinking ‘what’s the best approach,’” Burden says. They may even choose to fight the predators rather than flee, she adds. “In some parts of the world, people use them as guard animals against things like coyotes and wolves.”
Scientists believe that domestic donkeys take their origin from Equus africanus or African wild ass, originally roaming where Kenya, Ethiopia and Eritrea are today. Having evolved in the harsh and arid mountainous terrains where food and water were scarce, they are extremely adaptable and hardy. Research finds that they can go without water for 72 hours and then drink their fill without any negative consequences. Their big jaws let them chew tough desert shrubs, which horses can’t exist on. Their large ears help dissipate heat. Their little upright hooves are a perfect fit for the uneven rocky or other dangerous grounds. Accustomed to the mountain desert climate with hot days and cold nights, they don’t mind temperature flux.
“The donkey is the most supremely adapted animal to deal with hostile conditions,” Burden says. “They can survive on much lower nutritional quality food than a cow, sheep or horse. That’s why communities living in some of the most inhospitable places will often have donkeys with them.” And that’s why losing a donkey to an illegal skin trade can devastate a family in places like Eritrea. Suddenly everything from water to firewood to produce must be carried by family members—and often women.

Workers unloading donkeys at the Shinyanga slaughterhouse in Tanzania. Fearing a future in which donkeys go extinct, scientists have found ways to cryopreserve a donkey embryo in liquid nitrogen.
TAHUCHA
One can imagine a time when worldwide donkey populations may dwindle to the point that they would need to be restored. That includes their genetic variability too. That’s where the frozen embryos may come in handy. We may be able to use them to increase the genetic variability of donkeys, which will be especially important if they get closer to extinction, Gambini says. His team had already created frozen embryos for horses and zebras, an idea similar to a seed bank. “We call this concept the Frozen Zoo.”
Creating donkey embryos proved much harder than those of zebras and horses. To improve chances of fertilization, Gambini used the intracytoplasmic sperm injection or ICSI, in which he employed a tiny needle called a micropipette to inject a donkey sperm into an egg. That was a step above the traditional IVF method, in which the egg and a sperm are left floating in a test tube together. The injection took, but during the incubating week, one after the other, the embryos stopped dividing. Finally, on day seven, Gambini finally spotted the exact sight he was hoping to see. One of the embryos developed into a burgeoning ball of cells.
“That stage is called a blastocyst,” Gambini says. The clump of cells had a lot of fluids mixed within them, which indicated that they were finally developing into a viable embryo. “When we see a blastocyst, we know we can transfer that into a female.” He was so excited he immediately called all his collaborators to tell them the good news, which they later published in the journal of Theriogenology.
The one and only embryo to reach that stage, the blastocyst was cryopreserved in liquid nitrogen. The team is waiting for the next breeding season to see if a female donkey may carry it to term and give birth to a healthy foal. Gambini’s team is hoping to polish the process and create more embryos. “It’s our weapon in the conservation ass-enal,” he says.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Too much of this ingredient leads to autoimmune diseases, new research shows. Here's how to cut back.
Scientists are looking at how salt affects our cells, and they're finding more reasons to avoid htoo much of it.
For more than a century, doctors have warned that too much salt in your diet can lead to high blood pressure, heart disease and stroke - and many of the reasons for these effects are well known. But recently scientists have been looking deeper, into the cellular level, and they are finding additional reasons to minimize sodium intake; it is bad for immune cells, creating patterns of gene expression and activity seen in a variety of autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, and type-1 diabetes.
Salt is a major part of the ocean from which life evolved on this planet. We carry that legacy in our blood, which tastes salty. It is an important element for conducting electrical signals along nerves and balancing water and metabolites transported throughout our bodies. We need to consume about 500 milligrams of salt each day to maintain these functions, more with exercise and heavy sweating as that is a major way the body loses salt. The problem is that most Americans eating a modern western diet consume about 3400 milligrams, 1.5 teaspoons per day.
Evidence has been accumulating over the last few years that elevated levels of sodium can be harmful to at least some types of immune cells. The first signal came in monocytes, which are immune cells that travel to various tissues in the body, where some of them turn into macrophages, a subset of white blood cells that can directly kill microorganisms and make chemical signals that bring other types of immune cells into play.
Two years ago, Dominik N. Müller from the Max-Delbrueck-Center in Berlin, Germany and Markus Kleinewietfeld, an immunologist at Hasselt University in Belgium, ran a study where they fed people pizza and then measured their immune cell function. “We saw that in any monocytes, metabolic function was down, even after a single salty meal,” Kleinewietfeld says. It seemed to be the cellular equivalent of the sluggish feeling we get after eating too much. The cells were able to recover but more research is needed to answer questions about what dose of sodium causes impairment, how long the damage lasts, and whether there is a cumulative effect of salt toxicity.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations.
The latest series of experiments focused on a type of T cell called T regulatory cells, or Tregs. Most T cells release inflammatory mediators to fight pathogens and, once that job is done, Tregs come along to calm down their hyperactive brethren. Failure to do so can result in continued inflammation and possibly autoimmune diseases.
In the lab, Kleinewietfeld and his large team of international collaborators saw that high levels of sodium had a huge effect on Tregs, upregulating 1250 genes and downregulating an additional 1380 genes so that they looked similar to patterns of gene expression seen in autoimmune diseases.
Digging deeper, they found that sodium affected mitochondria, the tiny organelles inside of cells that produce much of its energy. The sodium was interfering with how the mitochondria use oxygen, which resulted in increased levels of an unstable form of oxygen that can damage cell function. The researchers injected those damaged Tregs into mice and found that they impaired the animals' immune function, allowing the inflammation to continue rather than shutting it down.
That finding dovetailed nicely with a 2019 paper in Nature from Navdeep Chandel's lab at Northwestern University, which showed in mice that inhibiting the mitochondrial use of oxygen reduced the ability of Tregs to regulate other T cells. “Mitochondria were controlling directly the immunosuppressive program, they were this master regulator tuning the right amount of genes to give you proper immunosuppression,” Chandel said. “And if you lose that function, then you get autoimmunity.”
Kleinewietfeld's team studied the Treg cells of humans and found that sodium can similarly decrease mitochondrial use of oxygen and immunosuppressive activity. “I would have never predicted that myself,” Chandel says, but now researchers can look at the mitochondria of patients with autoimmune disease and see if their gene expression also changes under high salt conditions. He sees the link between the patterns of gene expression in Tregs generated by high salt exposure and those patterns seen in autoimmune diseases, but he is cautious about claiming a causal effect.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations. He says a high salt diet could also have an indirect effect on immune function through the way it affects the gut microbiome and the molecules made by microbes when they break down food. But the research results are too preliminary to say that for sure, much less parse out the role of salt compared with other possible factors. “It is still an exciting journey to try to understand this field,” he says.
Additionally, it is difficult to say precisely how this research in animals and human cell cultures will translate into a whole human body. Individual differences in genetics can affect how the body absorbs, transports, and gets rid of sodium, such that some people are more sensitive to salt than are others.
So how should people apply these research findings to daily life?
Salt is obvious when we sprinkle it on at the table or eat tasty things like potato chips, but we may be unaware of sodium hidden in packaged foods. That's because salt is an easy and cheap way to boost the flavor of foods. And if we do read the labeled salt content on a package, we focus on the number for a single serving, but then eat more than that.
Last September, the U.S. Food and Drug Administration (FDA) began a process to update labels on the content of food, including what is meant by the word “healthy” and how food manufacturers can use the term. Many in the food industry are resisting those proposed changes.
Chandel cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker.
Until labels are updated, it would be prudent to try to reduce sodium intake by cutting down on packaged foods while making your own food at home, where you know just how much salt has been added. The Mayo Clinic offers guidance on how to become more aware of the sodium in your diet and eat less of it.
Chandel thinks many people will struggle with minimizing salt in their diets. It’s similar to the challenge of eating less sugar, in that the body craves both, and it is difficult to fight that. He cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker. “Dietary antioxidants have failed in just about every clinical trial, yet the public continues to take them,” Chandel says. But he is optimistic that research will lead us to a better understanding of how Tregs function, and uncover new targets for treating autoimmune diseases.

