Can Spare Parts from Pigs Solve Our Organ Shortage?
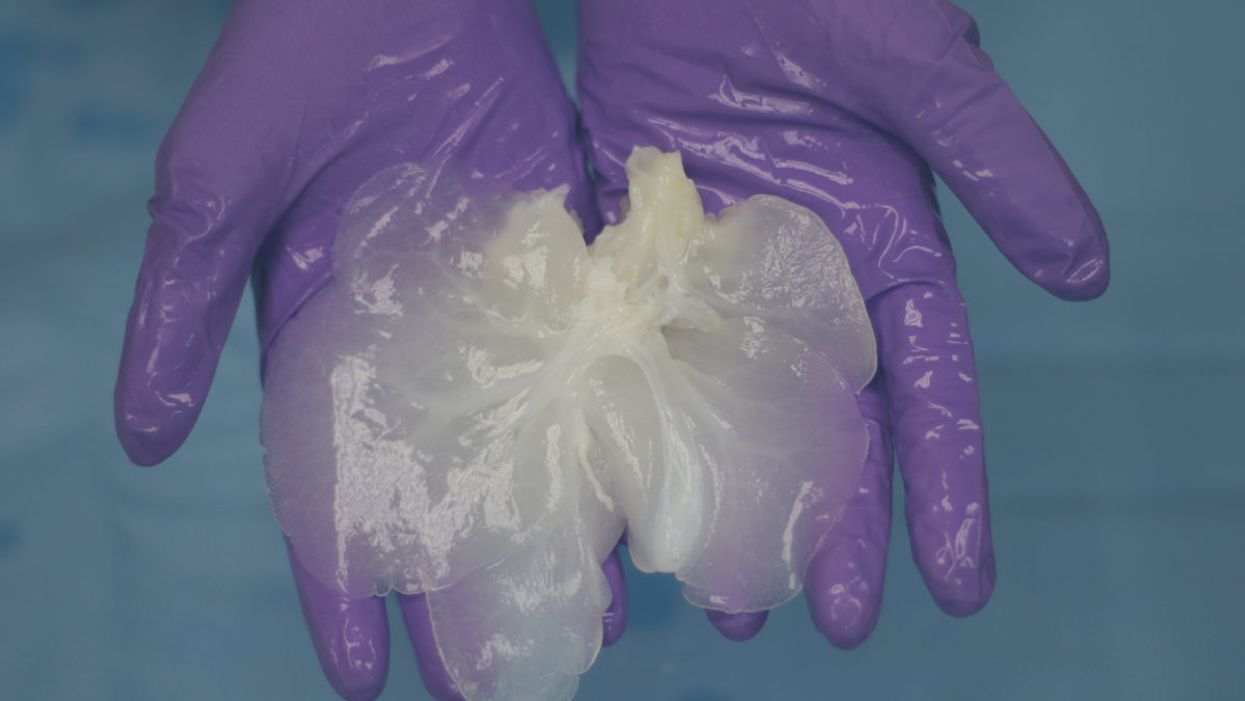
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."
The Friday Five: Artificial DNA Could Give Cancer the Hook
In this week's Friday Five, artificial DNA could give cancer the hook. Plus, research suggests that this daily practice can improve relationships, social media could be good for finding factual information, injecting a gel may speed up recovery from injuries, and a blood pressure medicine is promising for a long healthy life.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- Artificial DNA gives cancer the hook
- This daily practice could improve relationships
- Can social media handle the truth?
- Injecting a gel could speed up recovery
- A blood pressure medicine for a long healthy life
9 Tips for Online Mental Health Therapy
Research shows that, for most patients, online therapy offers the same benefits as in-person therapy, yet many people still resist it. A behavioral scientist explains how you can use it to improve mental health.
Telehealth offers a vast improvement in access and convenience to all sorts of medical services, and online therapy for mental health is one of the most promising case studies for telehealth. With many online therapy options available, you can choose whatever works best for you. Yet many people are hesitant about using online therapy. Even if they do give it a try, they often don’t know how to make the most effective use of this treatment modality.
Why do so many feel uncertain about online therapy? A major reason stems from its novelty. Humans are creatures of habit, prone to falling for what behavioral scientists like myself call the status quo bias, a predisposition to stick to traditional practices and behaviors. Many people reject innovative solutions even when they would be helpful. Thus, while teletherapy was available long before the pandemic, and might have fit the needs of many potential clients, relatively few took advantage of this option.
Even when we do try new methodologies, we often don’t do so effectively, because we cling to the same approaches that worked in previous situations. Scientists call this behavior functional fixedness. It’s kind of like the saying about the hammer-nail syndrome: “when you have a hammer, everything looks like a nail.”
These two mental blindspots, the status quo bias and functional fixedness, impact decision making in many areas of life. Fortunately, recent research has shown effective and pragmatic strategies to defeat these dangerous errors in judgment. The nine tips below will help you make the best decisions to get effective online therapy, based on the latest research.
Trust the science of online therapy
Extensive research shows that, for most patients, online therapy offers the same benefits as in-person therapy.
For instance, a 2014 study in the Journal of Affective Disorders reported that online treatment proved just as effective as face-to-face treatment for depression. A 2018 study, published in Journal of Psychological Disorders, found that online cognitive behavioral therapy, or CBT, was just as effective as face-to-face treatment for major depression, panic disorder, social anxiety disorder, and generalized anxiety disorder. And a 2014 study in Behaviour Research and Therapy discovered that online CBT proved effective in treating anxiety disorders, and helped lower costs of treatment.
During the forced teletherapy of COVID, therapists worried that those with serious mental health conditions would be less likely to convert to teletherapy. Yet research published in Counselling Psychology Quarterly has helped to alleviate that concern. It found that those with schizophrenia, bipolar disorder, severe depression, PTSD, and even suicidality converted to teletherapy at about the same rate as those with less severe mental health challenges.
Yet teletherapy may not be for everyone. For example, adolescents had the most varied response to teletherapy, according to a 2020 study in Family Process. Some adapted quickly and easily, while others found it awkward and anxiety-inducing. On the whole, children with trauma respond worse to online therapy, per a 2020 study in Child Abuse & Neglect. The treatment of mental health issues can sometimes require in-person interactions, such as the use of eye movement desensitization and reprocessing to treat post-traumatic stress disorder. And according to a 2020 study from the Journal of Humanistic Psychology, online therapy may not be as effective for those suffering from loneliness.
Leverage the strengths of online therapy
Online therapy is much more accessible than in-person therapy for those with a decent internet connection, webcam, mic, and digital skills. You don’t have to commute to your therapist’s office, wasting money and time. You can take much less medical leave from work, saving you money and hassle with your boss. If you live in a sparsely populated area, online therapy could allow you to access many specialized kinds of therapy that isn’t accessible locally.
Online options are much quicker compared to the long waiting lines for in-person therapy. You also have much more convenient scheduling options. And you won’t have to worry about running into someone you know in the waiting room. Online therapy is easier to conceal from others and reduces stigma. Many patients may feel more comfortable and open to sharing in the privacy and comfort of their own home.
You can use a variety of communication tools suited to your needs at any given time. Video can be used to start a relationship with a therapist and have more intense and nuanced discussions, but can be draining, especially for those with social anxiety. Voice-only may work well for less intense discussions. Email offers a useful option for long-form, well-thought-out messages. Texting is useful for quick, real-time questions, answers, and reinforcement.
Plus, online therapy is often cheaper than in-person therapy. In the midst of COVID, many insurance providers have decided to cover online therapy.
Address the weaknesses
One weakness is the requirement for appropriate technology and skills to engage in online therapy. Another is the difficulty of forming a close therapeutic relationship with your therapist. You won’t be able to communicate non-verbals as fully and the therapist will not be able to read you as well, requiring you to be more deliberate in how you express yourself.
Another important issue is that online therapy is subject to less government oversight compared to the in-person approach, which is regulated in each state, providing a baseline of quality control. As a result, you have to do more research on the providers that offer online therapy to make sure they’re reputable, use only licensed therapists, and have a clear and transparent pay structure.
Be intentional about advocating for yourself
Figure out what kind of goals you want to achieve. Consider how, within the context of your goals, you can leverage the benefits of online therapy while addressing the weaknesses. Write down and commit to achieving your goals. Remember, you need to be your own advocate, especially in the less regulated space of online therapy, so focus on being proactive in achieving your goals.
Develop your Hero’s Journey
Because online therapy can occur at various times of day through videos calls, emails and text, it might feel more open-ended and less organized, which can have advantages and disadvantages. One way you can give it more structure is to ground these interactions in the story of your self-improvement. Our minds perceive the world through narratives. Create a story of how you’ll get from where you are to where you want to go, meaning your goals.
A good template to use is the Hero’s Journey. Start the narrative with where you are, and what caused you to seek therapy. Write about the obstacles you will need to overcome, and the kind of help from a therapist that you’ll need in the process. Then, describe the final end state: how will you be better off after this journey, including what you will have learned.
Especially in online therapy, you need to be on top of things. Too many people let the therapist manage the treatment plan. As you pursue your hero’s journey, another way to organize for success is to take notes on your progress, and reevaluate how you’re doing every month with your therapist.
Identify your ideal mentor
Since it’s more difficult to be confident about the quality of service providers in an online setting, you should identify in advance the traits of your desired therapist. Every Hero’s Journey involves a mentor figure who guides the protagonist through this journey. So who’s your ideal mentor? Write out their top 10 characteristics, from most to least important.
For example, you might want someone who is:
- Empathetic
- Caring
- Good listener
- Logical
- Direct
- Questioning
- Non-judgmental
- Organized
- Curious
- Flexible
That’s my list. Depending on what challenge you’re facing and your personality and preferences, you should make your own. Then, when you are matched with a therapist, evaluate how well they fit your ideal list.
Fail fast
When you first match with a therapist, try to fail fast. That means, instead of focusing on getting treatment, focus on figuring out if the therapist is a good match based on the traits you identified above. That will enable you to move on quickly if they’re not, and it’s very much worth it to figure that out early.
Tell them your goals, your story, and your vision of your ideal mentor. Ask them whether they think they are a match, and what kind of a treatment plan they would suggest based on the information you provided. And observe them yourself in your initial interactions, focusing on whether they’re a good match. Often, you’ll find that your initial vision of your ideal mentor is incomplete, and you’ll learn through doing therapy what kind of a therapist is the best fit for you.
Choose a small but meaningful subgoal to work on first
This small subgoal should be sufficient to be meaningful and impactful for improving your mental health, but not a big stretch for you to achieve. This subgoal should be a tool for you to use to evaluate whether the therapist is indeed a good fit for you. It will also help you evaluate whether the treatment plan makes sense, or whether it needs to be revised.
Know when to wrap things up
As you approach the end of your planned work and you see you’re reaching your goals, talk to the therapist about how to wrap up rather than letting things drag on for too long. You don’t want to become dependent on therapy: it’s meant to be a temporary intervention. Some less scrupulous therapists will insist that therapy should never end and we should all stay in therapy forever, and you want to avoid falling for this line. When you reach your goals, end your therapy, unless you discover a serious new reason to continue it. Still, it may be wise to set up occasional check-ins once every three to six months to make sure you’re staying on the right track.