Can You Trust Your Gut for Food Advice?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Which foods are actually healthy for your individual gut microbiome? Several companies are offering personalized dietary guidance based on your test results, but their answers in one experiment turned up with some conflicting advice.
I recently got on the scale to weigh myself, thinking I've got to eat better. With so many trendy diets today claiming to improve health, from Keto to Paleo to Whole30, it can be confusing to figure out what we should and shouldn't eat for optimal nutrition.
A number of companies are now selling the concept of "personalized" nutrition based on the genetic makeup of your individual gut bugs.
My next thought was: I've got to lose a few pounds.
Consider a weird factoid: In addition to my fat, skin, bone and muscle, I'm carrying around two or three pounds of straight-up bacteria. Like you, I am the host to trillions of micro-organisms that live in my gut and are collectively known as my microbiome. An explosion of research has occurred in the last decade to try to understand exactly how these microbial populations, which are unique to each of us, may influence our overall health and potentially even our brains and behavior.
Lots of mysteries still remain, but it is established that these "bugs" are crucial to keeping our body running smoothly, performing functions like stimulating the immune system, synthesizing important vitamins, and aiding digestion. The field of microbiome science is evolving rapidly, and a number of companies are now selling the concept of "personalized" nutrition based on the genetic makeup of your individual gut bugs. The two leading players are Viome and DayTwo, but the landscape includes the newly launched startup Onegevity Health and others like Thryve, which offers customized probiotic supplements in addition to dietary recommendations.
The idea has immediate appeal – if science could tell you exactly what to make for lunch and what to avoid, you could forget about the fad diets and go with your own bespoke food pyramid. Wondering if the promise might be too good to be true, I decided to perform my own experiment.
Last fall, I sent the identical fecal sample to both Viome (I paid $425, but the price has since dropped to $299) and DayTwo ($349). A couple of months later, both reports finally arrived, and I eagerly opened each app to compare their recommendations.
First, I examined my results from Viome, which was founded in 2016 in Cupertino, Calif., and declares without irony on its website that "conflicting food advice is now obsolete."
I learned I have "average" metabolic fitness and "average" inflammatory activity in my gut, which are scores that the company defines based on a proprietary algorithm. But I have "low" microbial richness, with only 62 active species of bacteria identified in my sample, compared with the mean of 157 in their test population. I also received a list of the specific species in my gut, with names like Lactococcus and Romboutsia.
But none of it meant anything to me without actionable food advice, so I clicked through to the Recommendations page and found a list of My Superfoods (cranberry, garlic, kale, salmon, turmeric, watermelon, and bone broth) and My Foods to Avoid (chickpeas, kombucha, lentils, and rice noodles). There was also a searchable database of many foods that had been categorized for me, like "bell pepper; minimize" and "beef; enjoy."
"I just don't think sufficient data is yet available to make reliable personalized dietary recommendations based on one's microbiome."
Next, I looked at my results from DayTwo, which was founded in 2015 from research out of the Weizmann Institute of Science in Israel, and whose pitch to consumers is, "Blood sugar made easy. The algorithm diet personalized to you."
This app had some notable differences. There was no result about my metabolic fitness, microbial richness, or list of the species in my sample. There was also no list of superfoods or foods to avoid. Instead, the app encouraged me to build a meal by searching for foods in their database and combining them in beneficial ways for my blood sugar. Two slices of whole wheat bread received a score of 2.7 out of 10 ("Avoid"), but if combined with one cup of large curd cottage cheese, the score improved to 6.8 ("Limit"), and if I added two hard-boiled eggs, the score went up to 7.5 ("Good").
Perusing my list of foods with "Excellent" scores, I noticed some troubling conflicts with the other app. Lentils, which had been a no-no according to Viome, received high marks from DayTwo. Ditto for Kombucha. My purported superfood of cranberry received low marks. Almonds got an almost perfect score (9.7) while Viome told me to minimize them. I found similarly contradictory advice for foods I regularly eat, including navel oranges, peanuts, pork, and beets.
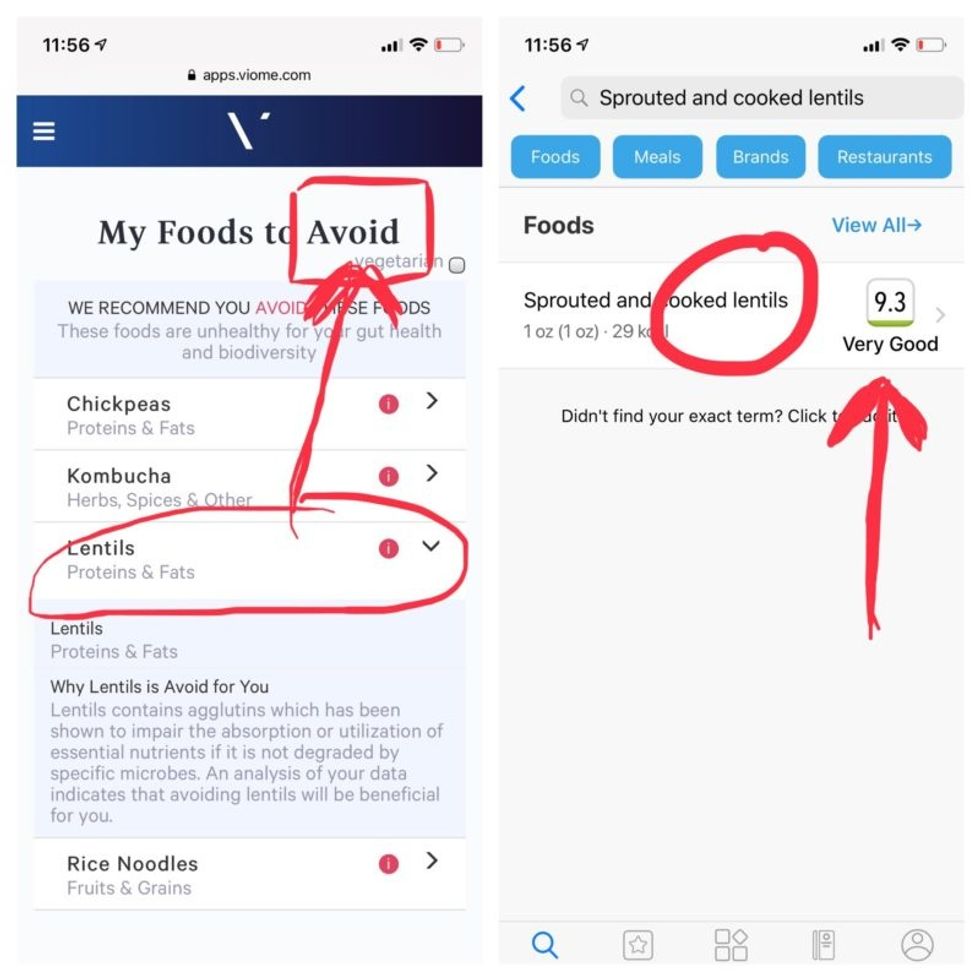
Contradictory dietary guidance that Kira Peikoff received from Viome (left) and DayTwo from an identical sample.
To be sure, there was some overlap. Both apps agreed on rice noodles (bad), chickpeas (bad), honey (bad), carrots (good), and avocado (good), among other foods.
But still, I was left scratching my head. Which set of recommendations should I trust, if either? And what did my results mean for the accuracy of this nascent field?
I called a couple of experts to find out.
"I have worked on the microbiome and nutrition for the last 20 years and I would be absolutely incapable of finding you evidence in the scientific literature that lentils have a detrimental effect based on the microbiome," said Dr. Jens Walter, an Associate Professor and chair for Nutrition, Microbes, and Gastrointestinal Health at the University of Alberta. "I just don't think sufficient data is yet available to make reliable personalized dietary recommendations based on one's microbiome. And even if they would have proprietary algorithms, at least one of them is not doing it right."
There is definite potential for personalized nutrition based on the microbiome, he said, but first, predictive models must be built and standardized, then linked to clinical endpoints, and tested in a large sample of healthy volunteers in order to enable extrapolations for the general population.
"It is mindboggling what you would need to do to make this work," he observed. "There are probably hundreds of relevant dietary compounds, then the microbiome has at least a hundred relevant species with a hundred or more relevant genes each, then you'd have to put all this together with relevant clinical outcomes. And there's a hundred-fold variation in that information between individuals."
However, Walter did acknowledge that the companies might be basing their algorithms on proprietary data that could potentially connect all the dots. I reached out to them to find out.
Amir Golan, the Chief Commercial Officer of DayTwo, told me, "It's important to emphasize this is a prediction, as the microbiome field is in a very early stage of research." But he added, "I believe we are the only company that has very solid science published in top journals and we can bring very actionable evidence and benefit to our uses."
He was referring to pioneering work out of the Weizmann Institute that was published in 2015 in the journal Cell, which logged the glycemic responses of 800 people in response to nearly 50,000 meals; adding information about the subjects' microbiomes enabled more accurate glycemic response predictions. Since then, Golan said, additional trials have been conducted, most recently with the Mayo Clinic, to duplicate the results, and other studies are ongoing whose results have not yet been published.
He also pointed out that the microbiome was merely one component that goes into building a client's profile, in addition to medical records, including blood glucose levels. (I provided my HbA1c levels, a measure of average blood sugar over the previous several months.)
"We are not saying we want to improve your gut microbiome. We provide a dynamic tool to help guide what you should eat to control your blood sugar and think about combinations," he said. "If you eat one thing, or with another, it will affect you in a different way."
Viome acknowledged that the two companies are taking very different approaches.
"DayTwo is primarily focused on the glycemic response," Naveen Jain, the CEO, told me. "If you can only eat butter for rest of your life, you will have no glycemic response but will probably die of a heart attack." He laughed. "Whereas we came from very different angle – what is happening inside the gut at a microbial level? When you eat food like spinach, how will that be metabolized in the gut? Will it produce the nutrients you need or cause inflammation?"
He said his team studied 1000 people who were on continuous glucose monitoring and fed them 45,000 meals, then built a proprietary data prediction model, looking at which microbes existed and how they actively broke down the food.
Jain pointed out that DayTwo sequences the DNA of the microbes, while Viome sequences the RNA – the active expression of DNA. That difference, in his opinion, is key to making accurate predictions.
"DNA is extremely stable, so when you eat any food and measure the DNA [in a fecal sample], you get all these false positives--you get DNA from plant food and meat, and you have no idea if those organisms are dead and simply transient, or actually exist. With RNA, you see what is actually alive in the gut."
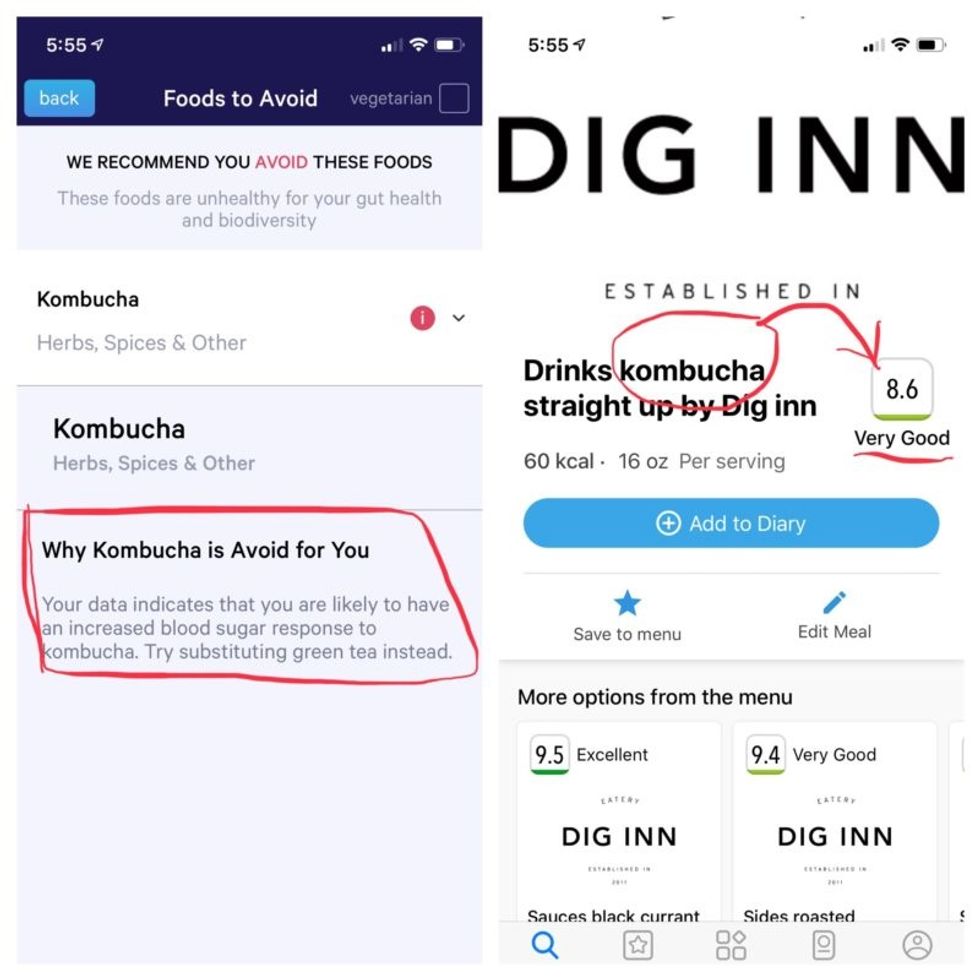
More contradictory food advice from Viome (left) and DayTwo.
Note that controversy exists over how it is possible with a fecal sample to effectively measure RNA, which degrades within minutes, though Jain said that his company has the technology to keep RNA stable for fourteen days.
Viome's approach, Jain maintains, is 90 percent accurate, based on as-yet unpublished data; a patent was filed just last week. DayTwo's approach is 66 percent accurate according to the latest published research.
Natasha Haskey, a registered dietician and doctoral student conducting research in the field of microbiome science and nutrition, is skeptical of both companies. "We can make broad statements, like eat more fruits and vegetables and fiber, but when it comes to specific foods, the science is just not there yet," she said. "I think there is a future, and we will be doing that someday, but not yet. Maybe we will be closer in ten years."
Professor Walter wholeheartedly agrees with Haskey, and suggested that if people want to eat a gut-healthy diet, they should focus on beneficial oils, fruits and vegetables, fish, a variety of whole grains, poultry and beans, and limit red meat and cheese, as well as avoid processed meats.
"These services are far over the tips of their science skis," Arthur Caplan, the founding head of New York University's Division of Medical Ethics, said in an email. "We simply don't know enough about the gut microbiome, its fluctuations and variability from person to person to support general [direct-to-consumer] testing. This is simply premature. We need standards for accuracy, specificity, and sensitivity, plus mandatory competent counseling for all such testing. They don't exist. Neither should DTC testing—yet."
Meanwhile, it's time for lunch. I close out my Viome and DayTwo apps and head to the kitchen to prepare a peanut butter sandwich. My gut tells me I'll be just fine.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Breakthrough therapies are breaking patients' banks. Key changes could improve access, experts say.
Single-treatment therapies are revolutionizing medicine. But insurers and patients wonder whether they can afford treatment and, if they can, whether the high costs are worthwhile.
CSL Behring’s new gene therapy for hemophilia, Hemgenix, costs $3.5 million for one treatment, but helps the body create substances that allow blood to clot. It appears to be a cure, eliminating the need for other treatments for many years at least.
Likewise, Novartis’s Kymriah mobilizes the body’s immune system to fight B-cell lymphoma, but at a cost $475,000. For patients who respond, it seems to offer years of life without the cancer progressing.
These single-treatment therapies are at the forefront of a new, bold era of medicine. Unfortunately, they also come with new, bold prices that leave insurers and patients wondering whether they can afford treatment and, if they can, whether the high costs are worthwhile.
“Most pharmaceutical leaders are there to improve and save people’s lives,” says Jeremy Levin, chairman and CEO of Ovid Therapeutics, and immediate past chairman of the Biotechnology Innovation Organization. If the therapeutics they develop are too expensive for payers to authorize, patients aren’t helped.
“The right to receive care and the right of pharmaceuticals developers to profit should never be at odds,” Levin stresses. And yet, sometimes they are.
Leigh Turner, executive director of the bioethics program, University of California, Irvine, notes this same tension between drug developers that are “seeking to maximize profits by charging as much as the market will bear for cell and gene therapy products and other medical interventions, and payers trying to control costs while also attempting to provide access to medical products with promising safety and efficacy profiles.”
Why Payers Balk
Health insurers can become skittish around extremely high prices, yet these therapies often accompany significant overall savings. For perspective, the estimated annual treatment cost for hemophilia exceeds $300,000. With Hemgenix, payers would break even after about 12 years.
But, in 12 years, will the patient still have that insurer? Therein lies the rub. U.S. payers, are used to a “pay-as-you-go” model, in which the lifetime costs of therapies typically are shared by multiple payers over many years, as patients change jobs. Single treatment therapeutics eliminate that cost-sharing ability.
"As long as formularies are based on profits to middlemen…Americans’ healthcare costs will continue to skyrocket,” says Patricia Goldsmith, the CEO of CancerCare.
“There is a phenomenally complex, bureaucratic reimbursement system that has grown, layer upon layer, during several decades,” Levin says. As medicine has innovated, payment systems haven’t kept up.
Therefore, biopharma companies begin working with insurance companies and their pharmacy benefit managers (PBMs), which act on an insurer’s behalf to decide which drugs to cover and by how much, early in the drug approval process. Their goal is to make sophisticated new drugs available while still earning a return on their investment.
New Payment Models
Pay-for-performance is one increasingly popular strategy, Turner says. “These models typically link payments to evidence generation and clinically significant outcomes.”
A biotech company called bluebird bio, for example, offers value-based pricing for Zynteglo, a $2.8 million possible cure for the rare blood disorder known as beta thalassaemia. It generally eliminates patients’ need for blood transfusions. The company is so sure it works that it will refund 80 percent of the cost of the therapy if patients need blood transfusions related to that condition within five years of being treated with Zynteglo.
In his February 2023 State of the Union speech, President Biden proposed three pilot programs to reduce drug costs. One of them, the Cell and Gene Therapy Access Model calls on the federal Centers for Medicare & Medicaid Services to establish outcomes-based agreements with manufacturers for certain cell and gene therapies.
A mortgage-style payment system is another, albeit rare, approach. Amortized payments spread the cost of treatments over decades, and let people change employers without losing their healthcare benefits.
Only about 14 percent of all drugs that enter clinical trials are approved by the FDA. Pharma companies, therefore, have an exigent need to earn a profit.
The new payment models that are being discussed aren’t solutions to high prices, says Bill Kramer, senior advisor for health policy at Purchaser Business Group on Health (PBGH), a nonprofit that seeks to lower health care costs. He points out that innovative pricing models, although well-intended, may distract from the real problem of high prices. They are attempts to “soften the blow. The best thing would be to charge a reasonable price to begin with,” he says.
Instead, he proposes making better use of research on cost and clinical effectiveness. The Institute for Clinical and Economic Review (ICER) conducts such research in the U.S., determining whether the benefits of specific drugs justify their proposed prices. ICER is an independent non-profit research institute. Its reports typically assess the degrees of improvement new therapies offer and suggest prices that would reflect that. “Publicizing that data is very important,” Kramer says. “Their results aren’t used to the extent they could and should be.” Pharmaceutical companies tend to price their therapies higher than ICER’s recommendations.
Drug Development Costs Soar
Drug developers have long pointed to the onerous costs of drug development as a reason for high prices.
A 2020 study found the average cost to bring a drug to market exceeded $1.1 billion, while other studies have estimated overall costs as high as $2.6 billion. The development timeframe is about 10 years. That’s because modern therapeutics target precise mechanisms to create better outcomes, but also have high failure rates. Only about 14 percent of all drugs that enter clinical trials are approved by the FDA. Pharma companies, therefore, have an exigent need to earn a profit.
Skewed Incentives Increase Costs
Pricing isn’t solely at the discretion of pharma companies, though. “What patients end up paying has much more to do with their PBMs than the actual price of the drug,” Patricia Goldsmith, CEO, CancerCare, says. Transparency is vital.
PBMs control patients’ access to therapies at three levels, through price negotiations, pricing tiers and pharmacy management.
When negotiating with drug manufacturers, Goldsmith says, “PBMs exchange a preferred spot on a formulary (the insurer’s or healthcare provider’s list of acceptable drugs) for cash-base rebates.” Unfortunately, 25 percent of the time, those rebates are not passed to insurers, according to the PBGH report.
Then, PBMs use pricing tiers to steer patients and physicians to certain drugs. For example, Kramer says, “Sometimes PBMs put a high-cost brand name drug in a preferred tier and a lower-cost competitor in a less preferred, higher-cost tier.” As the PBGH report elaborates, “(PBMs) are incentivized to include the highest-priced drugs…since both manufacturing rebates, as well as the administrative fees they charge…are calculated as a percentage of the drug’s price.
Finally, by steering patients to certain pharmacies, PBMs coordinate patients’ access to treatments, control patients’ out-of-pocket costs and receive management fees from the pharmacies.
Therefore, Goldsmith says, “As long as formularies are based on profits to middlemen…Americans’ healthcare costs will continue to skyrocket.”
Transparency into drug pricing will help curb costs, as will new payment strategies. What will make the most impact, however, may well be the development of a new reimbursement system designed to handle dramatic, breakthrough drugs. As Kramer says, “We need a better system to identify drugs that offer dramatic improvements in clinical care.”
In today's podcast episode, law professor Gaia Bernstein talks about the challenges of keeping control over our thoughts and actions, even when some powerful forces are pushing in the other direction.
Each afternoon, kids walk through my neighborhood, on their way back home from school, and almost all of them are walking alone, staring down at their phones. It's a troubling site. This daily parade of the zombie children just can’t bode well for the future.
That’s one reason I felt like Gaia Bernstein’s new book was talking directly to me. A law professor at Seton Hall, Gaia makes a strong argument that people are so addicted to tech at this point, we need some big, system level changes to social media platforms and other addictive technologies, instead of just blaming the individual and expecting them to fix these issues.
Gaia’s book is called Unwired: Gaining Control Over Addictive Technologies. It’s fascinating and I had a chance to talk with her about it for today’s podcast. At its heart, our conversation is really about how and whether we can maintain control over our thoughts and actions, even when some powerful forces are pushing in the other direction.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
We discuss the idea that, in certain situations, maybe it's not reasonable to expect that we’ll be able to enjoy personal freedom and autonomy. We also talk about how to be a good parent when it sometimes seems like our kids prefer to be raised by their iPads; so-called educational video games that actually don’t have anything to do with education; the root causes of tech addictions for people of all ages; and what kinds of changes we should be supporting.
Gaia is Seton’s Hall’s Technology, Privacy and Policy Professor of Law, as well as Co-Director of the Institute for Privacy Protection, and Co-Director of the Gibbons Institute of Law Science and Technology. She’s the founding director of the Institute for Privacy Protection. She created and spearheaded the Institute’s nationally recognized Outreach Program, which educated parents and students about technology overuse and privacy.
Professor Bernstein's scholarship has been published in leading law reviews including the law reviews of Vanderbilt, Boston College, Boston University, and U.C. Davis. Her work has been selected to the Stanford-Yale Junior Faculty Forum and received extensive media coverage. Gaia joined Seton Hall's faculty in 2004. Before that, she was a fellow at the Engelberg Center of Innovation Law & Policy and at the Information Law Institute of the New York University School of Law. She holds a J.S.D. from the New York University School of Law, an LL.M. from Harvard Law School, and a J.D. from Boston University.
Gaia’s work on this topic is groundbreaking I hope you’ll listen to the conversation and then consider pre-ordering her new book. It comes out on March 28.