New Study Shows “Living Drug” Can Provide a Lasting Cure for Cancer

A recent study by researchers at the University of Pennsylvania examined how CAR-T therapy helped Doug Olson beat a cancer death sentence for over a decade - and how it could work for more people.
Doug Olson was 49 when he was diagnosed with chronic lymphocytic leukemia, a blood cancer that strikes 21,000 Americans annually. Although the disease kills most patients within a decade, Olson’s case progressed more slowly, and courses of mild chemotherapy kept him healthy for 13 years. Then, when he was 62, the medication stopped working. The cancer had mutated, his doctor explained, becoming resistant to standard remedies. Harsher forms of chemo might buy him a few months, but their side effects would be debilitating. It was time to consider the treatment of last resort: a bone-marrow transplant.
Olson, a scientist who developed blood-testing instruments, knew the odds. There was only a 50 percent chance that a transplant would cure him. There was a 20 percent chance that the agonizing procedure—which involves destroying the patient’s marrow with chemo and radiation, then infusing his blood with donated stem cells—would kill him. If he survived, he would face the danger of graft-versus-host disease, in which the donor’s cells attack the recipient’s tissues. To prevent it, he would have to take immunosuppressant drugs, increasing the risk of infections. He could end up with pneumonia if one of his three grandchildren caught a sniffle. “I was being pushed into a corner,” Olson recalls, “with very little room to move.”
Soon afterward, however, his doctor revealed a possible escape route. He and some colleagues at the University of Pennsylvania’s Abramson Cancer Center were starting a clinical trial, he said, and Olson—still mostly symptom-free—might be a good candidate. The experimental treatment, known as CAR-T therapy, would use genetic engineering to turn his T lymphocytes (immune cells that guard against viruses and other pathogens) into a weapon against cancer.
In September 2010, technicians took some of Olson’s T cells to a laboratory, where they were programmed with new molecular marching orders and coaxed to multiply into an army of millions. When they were ready, a nurse inserted a catheter into his neck. At the turn of a valve, his soldiers returned home, ready to do battle.
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
Three weeks later, Olson was slammed with a 102-degree fever, nausea, and chills. The treatment had triggered two dangerous complications: cytokine release syndrome, in which immune chemicals inflame the patient’s tissues, and tumor lysis syndrome, in which toxins from dying cancer cells overwhelm the kidneys. But the crisis passed quickly, and the CAR-T cells fought on. A month after the infusion, the doctor delivered astounding news: “We can’t find any cancer in your body.”
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
An Unexpected Cure
In February 2022, the same cancer researchers reported a remarkable milestone: the trial’s first two patients had survived for more than a decade. Although Olson’s predecessor—a retired corrections officer named Bill Ludwig—died of COVID-19 complications in early 2021, both men had remained cancer-free. And the modified immune cells continued to patrol their territory, ready to kill suspected tumor cells the moment they arose.
“We can now conclude that CAR-T cells can actually cure patients with leukemia,” University of Pennsylvania immunologist Carl June, who spearheaded the development of the technique, told reporters. “We thought the cells would be gone in a month or two. The fact that they’ve survived 10 years is a major surprise.”
Even before the announcement, it was clear that CAR-T therapy could win a lasting reprieve for many patients with cancers that were once a death sentence. Since the Food and Drug Administration approved June’s version (marketed as Kymriah) in 2017, the agency has greenlighted five more such treatments for various types of leukemia, lymphoma, and myeloma. “Every single day, I take care of patients who would previously have been told they had no options,” says Rayne Rouce, a pediatric hematologist/oncologist at Texas Children’s Cancer Center. “Now we not only have a treatment option for those patients, but one that could potentially be the last therapy for their cancer that they’ll ever have to receive.”

Immunologist Carl June, middle, spearheaded development of the CAR-T therapy that gave patients Bill Ludwig, left, and Doug Olson, right, a lengthy reprieve on their terminal cancer diagnoses.
Penn Medicine
Yet the CAR-T approach doesn’t help everyone. So far, it has only shown success for blood cancers—and for those, the overall remission rate is 30 to 40 percent. “When it works, it works extraordinarily well,” says Olson’s former doctor, David Porter, director of Penn’s blood and bone marrow transplant program. “It’s important to know why it works, but it’s equally important to know why it doesn’t—and how we can fix that.”
The team’s study, published in the journal Nature, offers a wealth of data on what worked for these two patients. It may also hold clues for how to make the therapy effective for more people.
Building a Better T Cell
Carl June didn’t set out to cure cancer, but his serendipitous career path—and a personal tragedy—helped him achieve insights that had eluded other researchers. In 1971, hoping to avoid combat in Vietnam, he applied to the U.S. Naval Academy in Annapolis, Maryland. June showed a knack for biology, so the Navy sent him on to Baylor College of Medicine. He fell in love with immunology during a fellowship researching malaria vaccines in Switzerland. Later, the Navy deployed him to the Fred Hutchinson Cancer Research Center in Seattle to study bone marrow transplantation.
There, June became part of the first research team to learn how to culture T cells efficiently in a lab. After moving on to the National Naval Medical Center in the ’80s, he used that knowledge to combat the newly emerging AIDS epidemic. HIV, the virus that causes the disease, invades T cells and eventually destroys them. June and his post-doc Bruce Levine developed a method to restore patients’ depleted cell populations, using tiny magnetic beads to deliver growth-stimulating proteins. Infused into the body, the new T cells effectively boosted immune function.
In 1999, after leaving the Navy, June joined the University of Pennsylvania. His wife, who’d been diagnosed with ovarian cancer, died two years later, leaving three young children. “I had not known what it was like to be on the other side of the bed,” he recalls. Watching her suffer through grueling but futile chemotherapy, followed by an unsuccessful bone-marrow transplant, he resolved to focus on finding better cancer treatments. He started with leukemia—a family of diseases in which mutant white blood cells proliferate in the marrow.
Cancer is highly skilled at slipping through the immune system’s defenses. T cells, for example, detect pathogens by latching onto them with receptors designed to recognize foreign proteins. Leukemia cells evade detection, in part, by masquerading as normal white blood cells—that is, as part of the immune system itself.
June planned to use a viral vector no one had tried before: HIV.
To June, chimeric antigen receptor (CAR) T cells looked like a promising tool for unmasking and destroying the impostors. Developed in the early ’90s, these cells could be programmed to identify a target protein, and to kill any pathogen that displayed it. To do the programming, you spliced together snippets of DNA and inserted them into a disabled virus. Next, you removed some of the patient’s T cells and infected them with the virus, which genetically hijacked its new hosts—instructing them to find and slay the patient’s particular type of cancer cells. When the T cells multiplied, their descendants carried the new genetic code. You then infused those modified cells into the patient, where they went to war against their designated enemy.
Or that’s what happened in theory. Many scientists had tried to develop therapies using CAR-T cells, but none had succeeded. Although the technique worked in lab animals, the cells either died out or lost their potency in humans.
But June had the advantage of his years nurturing T cells for AIDS patients, as well as the technology he’d developed with Levine (who’d followed him to Penn with other team members). He also planned to use a viral vector no one had tried before: HIV, which had evolved to thrive in human T cells and could be altered to avoid causing disease. By the summer of 2010, he was ready to test CAR-T therapy against chronic lymphocytic leukemia (CLL), the most common form of the disease in adults.
Three patients signed up for the trial, including Doug Olson and Bill Ludwig. A portion of each man’s T cells were reprogrammed to detect a protein found only on B lymphocytes, the type of white blood cells affected by CLL. Their genetic instructions ordered them to destroy any cell carrying the protein, known as CD19, and to multiply whenever they encountered one. This meant the patients would forfeit all their B cells, not just cancerous ones—but regular injections of gamma globulins (a cocktail of antibodies) would make up for the loss.
After being infused with the CAR-T cells, all three men suffered high fevers and potentially life-threatening inflammation, but all pulled through without lasting damage. The third patient experienced a partial remission and survived for eight months. Olson and Ludwig were cured.
Learning What Works
Since those first infusions, researchers have developed reliable ways to prevent or treat the side effects of CAR-T therapy, greatly reducing its risks. They’ve also been experimenting with combination therapies—pairing CAR-T with chemo, cancer vaccines, and immunotherapy drugs called checkpoint inhibitors—to improve its success rate. But CAR-T cells are still ineffective for at least 60 percent of blood cancer patients. And they remain in the experimental stage for solid tumors (including pancreatic cancer, mesothelioma, and glioblastoma), whose greater complexity make them harder to attack.
The new Nature study offers clues that could fuel further advances. The Penn team “profiled these cells at a level where we can almost say, ‘These are the characteristics that a T cell would need to survive 10 years,’” says Rouce, the physician at Texas Children’s Cancer Center.
One surprising finding involves how CAR-T cells change in the body over time. At first, those that Olson and Ludwig received showed the hallmarks of “killer” T-cells (also known as CD8 cells)—highly active lymphocytes bent on exterminating every tumor cell in sight. After several months, however, the population shifted toward “helper” T-cells (or CD4s), which aid in forming long-term immune memory but are normally incapable of direct aggression. Over the years, the numbers swung back and forth, until only helper cells remained. Those cells showed markers suggesting they were too exhausted to function—but in the lab, they were able not only to recognize but to destroy cancer cells.
June and his team suspect that those tired-looking helper cells had enough oomph to kill off any B cells Olson and Ludwig made, keeping the pair’s cancers permanently at bay. If so, that could prompt new approaches to selecting cells for CAR-T therapy. Maybe starting with a mix of cell types—not only CD8s, but CD4s and other varieties—would work better than using CD8s alone. Or perhaps inducing changes in cell populations at different times would help.
Another potential avenue for improvement is starting with healthier cells. Evidence from this and other trials hints that patients whose T cells are more robust to begin with respond better when their cells are used in CAR-T therapy. The Penn team recently completed a clinical trial in which CLL patients were treated with ibrutinib—a drug that enhances T-cell function—before their CAR-T cells were manufactured. The response rate, says David Porter, was “very high,” with most patients remaining cancer-free a year after being infused with the souped-up cells.
Such approaches, he adds, are essential to achieving the next phase in CAR-T therapy: “Getting it to work not just in more people, but in everybody.”

Doug Olson enjoys nature - and having a future.
Penn Medicine
To grasp what that could mean, it helps to talk with Doug Olson, who’s now 75. In the years since his infusion, he has watched his four children forge careers, and his grandkids reach their teens. He has built a business and enjoyed the rewards of semi-retirement. He’s done volunteer and advocacy work for cancer patients, run half-marathons, sailed the Caribbean, and ridden his bike along the sun-dappled roads of Silicon Valley, his current home.
And in his spare moments, he has just sat there feeling grateful. “You don’t really appreciate the effect of having a lethal disease until it’s not there anymore,” he says. “The world looks different when you have a future.”
The Friday Five: How to exercise for cancer prevention
How to exercise for cancer prevention. Plus, a device that brings relief to back pain, ingredients for reducing Alzheimer's risk, the world's oldest disease could make you young again, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- How to exercise for cancer prevention
- A device that brings relief to back pain
- Ingredients for reducing Alzheimer's risk
- Is the world's oldest disease the fountain of youth?
- Scared of crossing bridges? Your phone can help
New approach to brain health is sparking memories
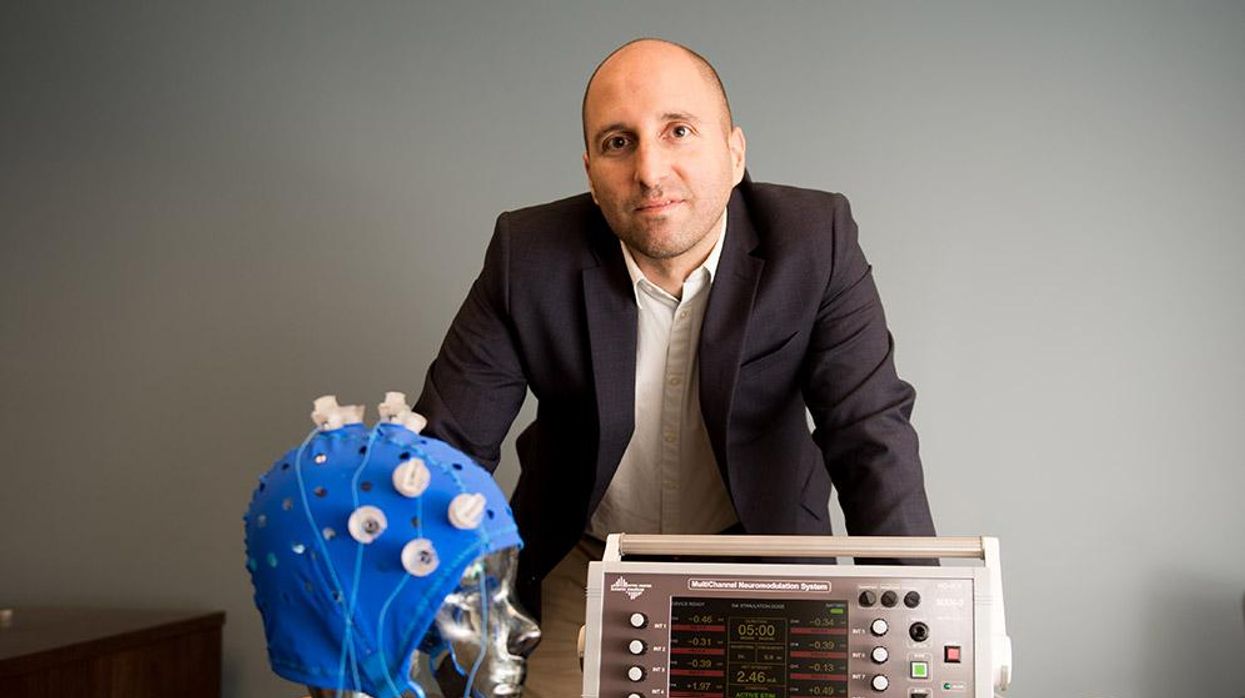
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”