Study Shows “Living Drug” Can Provide a Lasting Cure for Cancer

A recent study by researchers at the University of Pennsylvania examined how CAR-T therapy helped Doug Olson beat a cancer death sentence for over a decade - and how it could work for more people.
Doug Olson was 49 when he was diagnosed with chronic lymphocytic leukemia, a blood cancer that strikes 21,000 Americans annually. Although the disease kills most patients within a decade, Olson’s case progressed more slowly, and courses of mild chemotherapy kept him healthy for 13 years. Then, when he was 62, the medication stopped working. The cancer had mutated, his doctor explained, becoming resistant to standard remedies. Harsher forms of chemo might buy him a few months, but their side effects would be debilitating. It was time to consider the treatment of last resort: a bone-marrow transplant.
Olson, a scientist who developed blood-testing instruments, knew the odds. There was only a 50 percent chance that a transplant would cure him. There was a 20 percent chance that the agonizing procedure—which involves destroying the patient’s marrow with chemo and radiation, then infusing his blood with donated stem cells—would kill him. If he survived, he would face the danger of graft-versus-host disease, in which the donor’s cells attack the recipient’s tissues. To prevent it, he would have to take immunosuppressant drugs, increasing the risk of infections. He could end up with pneumonia if one of his three grandchildren caught a sniffle. “I was being pushed into a corner,” Olson recalls, “with very little room to move.”
Soon afterward, however, his doctor revealed a possible escape route. He and some colleagues at the University of Pennsylvania’s Abramson Cancer Center were starting a clinical trial, he said, and Olson—still mostly symptom-free—might be a good candidate. The experimental treatment, known as CAR-T therapy, would use genetic engineering to turn his T lymphocytes (immune cells that guard against viruses and other pathogens) into a weapon against cancer.
In September 2010, technicians took some of Olson’s T cells to a laboratory, where they were programmed with new molecular marching orders and coaxed to multiply into an army of millions. When they were ready, a nurse inserted a catheter into his neck. At the turn of a valve, his soldiers returned home, ready to do battle.
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
Three weeks later, Olson was slammed with a 102-degree fever, nausea, and chills. The treatment had triggered two dangerous complications: cytokine release syndrome, in which immune chemicals inflame the patient’s tissues, and tumor lysis syndrome, in which toxins from dying cancer cells overwhelm the kidneys. But the crisis passed quickly, and the CAR-T cells fought on. A month after the infusion, the doctor delivered astounding news: “We can’t find any cancer in your body.”
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
An Unexpected Cure
In February 2022, the same cancer researchers reported a remarkable milestone: the trial’s first two patients had survived for more than a decade. Although Olson’s predecessor—a retired corrections officer named Bill Ludwig—died of COVID-19 complications in early 2021, both men had remained cancer-free. And the modified immune cells continued to patrol their territory, ready to kill suspected tumor cells the moment they arose.
“We can now conclude that CAR-T cells can actually cure patients with leukemia,” University of Pennsylvania immunologist Carl June, who spearheaded the development of the technique, told reporters. “We thought the cells would be gone in a month or two. The fact that they’ve survived 10 years is a major surprise.”
Even before the announcement, it was clear that CAR-T therapy could win a lasting reprieve for many patients with cancers that were once a death sentence. Since the Food and Drug Administration approved June’s version (marketed as Kymriah) in 2017, the agency has greenlighted five more such treatments for various types of leukemia, lymphoma, and myeloma. “Every single day, I take care of patients who would previously have been told they had no options,” says Rayne Rouce, a pediatric hematologist/oncologist at Texas Children’s Cancer Center. “Now we not only have a treatment option for those patients, but one that could potentially be the last therapy for their cancer that they’ll ever have to receive.”

Immunologist Carl June, middle, spearheaded development of the CAR-T therapy that gave patients Bill Ludwig, left, and Doug Olson, right, a lengthy reprieve on their terminal cancer diagnoses.
Penn Medicine
Yet the CAR-T approach doesn’t help everyone. So far, it has only shown success for blood cancers—and for those, the overall remission rate is 30 to 40 percent. “When it works, it works extraordinarily well,” says Olson’s former doctor, David Porter, director of Penn’s blood and bone marrow transplant program. “It’s important to know why it works, but it’s equally important to know why it doesn’t—and how we can fix that.”
The team’s study, published in the journal Nature, offers a wealth of data on what worked for these two patients. It may also hold clues for how to make the therapy effective for more people.
Building a Better T Cell
Carl June didn’t set out to cure cancer, but his serendipitous career path—and a personal tragedy—helped him achieve insights that had eluded other researchers. In 1971, hoping to avoid combat in Vietnam, he applied to the U.S. Naval Academy in Annapolis, Maryland. June showed a knack for biology, so the Navy sent him on to Baylor College of Medicine. He fell in love with immunology during a fellowship researching malaria vaccines in Switzerland. Later, the Navy deployed him to the Fred Hutchinson Cancer Research Center in Seattle to study bone marrow transplantation.
There, June became part of the first research team to learn how to culture T cells efficiently in a lab. After moving on to the National Naval Medical Center in the ’80s, he used that knowledge to combat the newly emerging AIDS epidemic. HIV, the virus that causes the disease, invades T cells and eventually destroys them. June and his post-doc Bruce Levine developed a method to restore patients’ depleted cell populations, using tiny magnetic beads to deliver growth-stimulating proteins. Infused into the body, the new T cells effectively boosted immune function.
In 1999, after leaving the Navy, June joined the University of Pennsylvania. His wife, who’d been diagnosed with ovarian cancer, died two years later, leaving three young children. “I had not known what it was like to be on the other side of the bed,” he recalls. Watching her suffer through grueling but futile chemotherapy, followed by an unsuccessful bone-marrow transplant, he resolved to focus on finding better cancer treatments. He started with leukemia—a family of diseases in which mutant white blood cells proliferate in the marrow.
Cancer is highly skilled at slipping through the immune system’s defenses. T cells, for example, detect pathogens by latching onto them with receptors designed to recognize foreign proteins. Leukemia cells evade detection, in part, by masquerading as normal white blood cells—that is, as part of the immune system itself.
June planned to use a viral vector no one had tried before: HIV.
To June, chimeric antigen receptor (CAR) T cells looked like a promising tool for unmasking and destroying the impostors. Developed in the early ’90s, these cells could be programmed to identify a target protein, and to kill any pathogen that displayed it. To do the programming, you spliced together snippets of DNA and inserted them into a disabled virus. Next, you removed some of the patient’s T cells and infected them with the virus, which genetically hijacked its new hosts—instructing them to find and slay the patient’s particular type of cancer cells. When the T cells multiplied, their descendants carried the new genetic code. You then infused those modified cells into the patient, where they went to war against their designated enemy.
Or that’s what happened in theory. Many scientists had tried to develop therapies using CAR-T cells, but none had succeeded. Although the technique worked in lab animals, the cells either died out or lost their potency in humans.
But June had the advantage of his years nurturing T cells for AIDS patients, as well as the technology he’d developed with Levine (who’d followed him to Penn with other team members). He also planned to use a viral vector no one had tried before: HIV, which had evolved to thrive in human T cells and could be altered to avoid causing disease. By the summer of 2010, he was ready to test CAR-T therapy against chronic lymphocytic leukemia (CLL), the most common form of the disease in adults.
Three patients signed up for the trial, including Doug Olson and Bill Ludwig. A portion of each man’s T cells were reprogrammed to detect a protein found only on B lymphocytes, the type of white blood cells affected by CLL. Their genetic instructions ordered them to destroy any cell carrying the protein, known as CD19, and to multiply whenever they encountered one. This meant the patients would forfeit all their B cells, not just cancerous ones—but regular injections of gamma globulins (a cocktail of antibodies) would make up for the loss.
After being infused with the CAR-T cells, all three men suffered high fevers and potentially life-threatening inflammation, but all pulled through without lasting damage. The third patient experienced a partial remission and survived for eight months. Olson and Ludwig were cured.
Learning What Works
Since those first infusions, researchers have developed reliable ways to prevent or treat the side effects of CAR-T therapy, greatly reducing its risks. They’ve also been experimenting with combination therapies—pairing CAR-T with chemo, cancer vaccines, and immunotherapy drugs called checkpoint inhibitors—to improve its success rate. But CAR-T cells are still ineffective for at least 60 percent of blood cancer patients. And they remain in the experimental stage for solid tumors (including pancreatic cancer, mesothelioma, and glioblastoma), whose greater complexity make them harder to attack.
The new Nature study offers clues that could fuel further advances. The Penn team “profiled these cells at a level where we can almost say, ‘These are the characteristics that a T cell would need to survive 10 years,’” says Rouce, the physician at Texas Children’s Cancer Center.
One surprising finding involves how CAR-T cells change in the body over time. At first, those that Olson and Ludwig received showed the hallmarks of “killer” T-cells (also known as CD8 cells)—highly active lymphocytes bent on exterminating every tumor cell in sight. After several months, however, the population shifted toward “helper” T-cells (or CD4s), which aid in forming long-term immune memory but are normally incapable of direct aggression. Over the years, the numbers swung back and forth, until only helper cells remained. Those cells showed markers suggesting they were too exhausted to function—but in the lab, they were able not only to recognize but to destroy cancer cells.
June and his team suspect that those tired-looking helper cells had enough oomph to kill off any B cells Olson and Ludwig made, keeping the pair’s cancers permanently at bay. If so, that could prompt new approaches to selecting cells for CAR-T therapy. Maybe starting with a mix of cell types—not only CD8s, but CD4s and other varieties—would work better than using CD8s alone. Or perhaps inducing changes in cell populations at different times would help.
Another potential avenue for improvement is starting with healthier cells. Evidence from this and other trials hints that patients whose T cells are more robust to begin with respond better when their cells are used in CAR-T therapy. The Penn team recently completed a clinical trial in which CLL patients were treated with ibrutinib—a drug that enhances T-cell function—before their CAR-T cells were manufactured. The response rate, says David Porter, was “very high,” with most patients remaining cancer-free a year after being infused with the souped-up cells.
Such approaches, he adds, are essential to achieving the next phase in CAR-T therapy: “Getting it to work not just in more people, but in everybody.”

Doug Olson enjoys nature - and having a future.
Penn Medicine
To grasp what that could mean, it helps to talk with Doug Olson, who’s now 75. In the years since his infusion, he has watched his four children forge careers, and his grandkids reach their teens. He has built a business and enjoyed the rewards of semi-retirement. He’s done volunteer and advocacy work for cancer patients, run half-marathons, sailed the Caribbean, and ridden his bike along the sun-dappled roads of Silicon Valley, his current home.
And in his spare moments, he has just sat there feeling grateful. “You don’t really appreciate the effect of having a lethal disease until it’s not there anymore,” he says. “The world looks different when you have a future.”
This article was first published on Leaps.org on March 24, 2022.
Out of Thin Air: A Fresh Solution to Farming’s Water Shortages
Dry, arid and remote farming regions are vulnerable to water shortages, but scientists are working on a promising new solution.
California has been plagued by perilous droughts for decades. Freshwater shortages have sparked raging wildfires and killed fruit and vegetable crops. And California is not alone in its danger of running out of water for farming; parts of the Southwest, including Texas, are battling severe drought conditions, according to the North American Drought Monitor. These two states account for 316,900 of the 2 million total U.S. farms.
But even as farming becomes more vulnerable due to water shortages, the world's demand for food is projected to increase 70 percent by 2050, according to Guihua Yu, an associate professor of materials science at The University of Texas at Austin.
"Water is the most limiting natural resource for agricultural production because of the freshwater shortage and enormous water consumption needed for irrigation," Yu said.
As scientists have searched for solutions, an alternative water supply has been hiding in plain sight: Water vapor in the atmosphere. It is abundant, available, and endlessly renewable, just waiting for the moment that technological innovation and necessity converged to make it fit for use. Now, new super-moisture-absorbent gels developed by Yu and a team of researchers can pull that moisture from the air and bring it into soil, potentially expanding the map of farmable land around the globe to dry and remote regions that suffer from water shortages.
"This opens up opportunities to turn those previously poor-quality or inhospitable lands to become useable and without need of centralized water and power supplies," Yu said.
A renewable source of freshwater
The hydrogels are a gelatin-like substance made from synthetic materials. The gels activate in cooler, humid overnight periods and draw water from the air. During a four-week experiment, Yu's team observed that soil with these gels provided enough water to support seed germination and plant growth without an additional liquid water supply. And the soil was able to maintain the moist environment for more than a month, according to Yu.
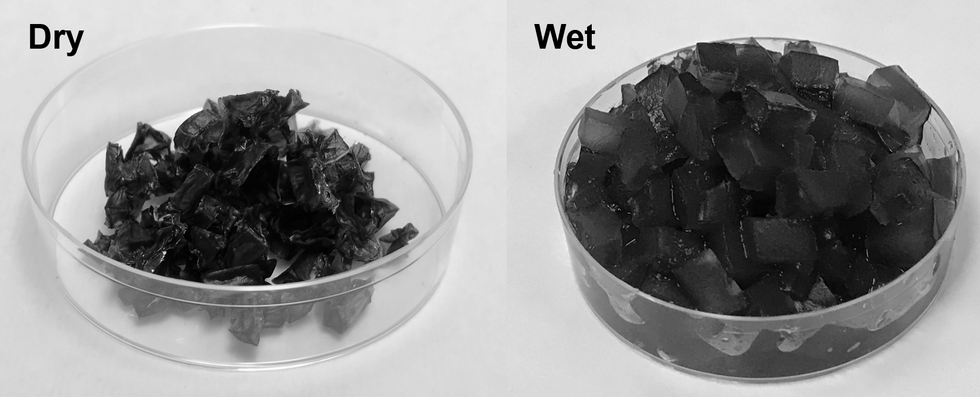
The super absorbent gels developed at the University of Texas at Austin.
Xingyi Zhou, UT Austin
"It is promising to liberate underdeveloped and drought areas from the long-distance water and power supplies for agricultural production," Yu said.
Crops also rely on fertilizer to maintain soil fertility and increase the production yield, but it is easily lost through leaching. Runoff increases agricultural costs and contributes to environmental pollution. The interaction between the gels and agrochemicals offer slow and controlled fertilizer release to maintain the balance between the root of the plant and the soil.
The possibilities are endless
Harvesting atmospheric water is exciting on multiple fronts. The super-moisture-absorbent gel can also be used for passively cooling solar panels. Solar radiation is the magic behind the process. Overnight, as temperatures cool, the gels absorb water hanging in the atmosphere. The moisture is stored inside the gels until the thermometer rises. Heat from the sun serves as the faucet that turns the gels on so they can release the stored water and cool down the panels. Effective cooling of the solar panels is important for sustainable long-term power generation.
In addition to agricultural uses and cooling for energy devices, atmospheric water harvesting technologies could even reach people's homes.
"They could be developed to enable easy access to drinking water through individual systems for household usage," Yu said.
Next steps
Yu and the team are now focused on affordability and developing practical applications for use. The goal is to optimize the gel materials to achieve higher levels of water uptake from the atmosphere.
"We are exploring different kinds of polymers and solar absorbers while exploring low-cost raw materials for production," Yu said.
The ability to transform atmospheric water vapor into a cheap and plentiful water source would be a game-changer. One day in the not-too-distant future, if climate change intensifies and droughts worsen, this innovation may become vital to our very survival.
On left, people excitedly line up for Salk's polio vaccine in 1957; on right, Joe Biden gets one of the COVID vaccines on December 21, 2020.
On the morning of April 12, 1955, newsrooms across the United States inked headlines onto newsprint: the Salk Polio vaccine was "safe, effective, and potent." This was long-awaited news. Americans had limped through decades of fear, unaware of what caused polio or how to cure it, faced with the disease's terrifying, visible power to paralyze and kill, particularly children.
The announcement of the polio vaccine was celebrated with noisy jubilation: church bells rang, factory whistles sounded, people wept in the streets. Within weeks, mass inoculation began as the nation put its faith in a vaccine that would end polio.
Today, most of us are blissfully ignorant of child polio deaths, making it easier to believe that we have not personally benefited from the development of vaccines. According to Dr. Steven Pinker, cognitive psychologist and author of the bestselling book Enlightenment Now, we've become blasé to the gifts of science. "The default expectation is not that disease is part of life and science is a godsend, but that health is the default, and any disease is some outrage," he says.
We're now in the early stages of another vaccine rollout, one we hope will end the ravages of the COVID-19 pandemic. And yet, the Pfizer, Moderna, and AstraZeneca vaccines are met with far greater hesitancy and skepticism than the polio vaccine was in the 50s.
In 2021, concerns over the speed and safety of vaccine development and technology plague this heroic global effort, but the roots of vaccine hesitancy run far deeper. Vaccine hesitancy has always existed in the U.S., even in the polio era, motivated in part by fears around "living virus" in a bad batch of vaccines produced by Cutter Laboratories in 1955. But in the last half century, we've witnessed seismic cultural shifts—loss of public trust, a rise in misinformation, heightened racial and socioeconomic inequality, and political polarization have all intensified vaccine-related fears and resistance. Making sense of how we got here may help us understand how to move forward.
The Rise and Fall of Public Trust
When the polio vaccine was released in 1955, "we were nearing an all-time high point in public trust," says Matt Baum, Harvard Kennedy School professor and lead author of several reports measuring public trust and vaccine confidence. Baum explains that the U.S. was experiencing a post-war boom following the Allied triumph in WWII, a popular Roosevelt presidency, and the rapid innovation that elevated the country to an international superpower.
The 1950s witnessed the emergence of nuclear technology, a space program, and unprecedented medical breakthroughs, adds Emily Brunson, Texas State University anthropologist and co-chair of the Working Group on Readying Populations for COVID-19 Vaccine. "Antibiotics were a game changer," she states. While before, people got sick with pneumonia for a month, suddenly they had access to pills that accelerated recovery.
During this period, science seemed to hold all the answers; people embraced the idea that we could "come to know the world with an absolute truth," Brunson explains. Doctors were portrayed as unquestioned gods, so Americans were primed to trust experts who told them the polio vaccine was safe.
"The emotional tone of the news has gone downward since the 1940s, and journalists consider it a professional responsibility to cover the negative."
That blind acceptance eroded in the 1960s and 70s as people came to understand that science can be inherently political. "Getting to an absolute truth works out great for white men, but these things affect people socially in radically different ways," Brunson says. As the culture began questioning the white, patriarchal biases of science, doctors lost their god-like status and experts were pushed off their pedestals. This trend continues with greater intensity today, as President Trump has led a campaign against experts and waged a war on science that began long before the pandemic.
The Shift in How We Consume Information
In the 1950s, the media created an informational consensus. The fundamental ideas the public consumed about the state of the world were unified. "People argued about the best solutions, but didn't fundamentally disagree on the factual baseline," says Baum. Indeed, the messaging around the polio vaccine was centralized and consistent, led by President Roosevelt's successful March of Dimes crusade. People of lower socioeconomic status with limited access to this information were less likely to have confidence in the vaccine, but most people consumed media that assured them of the vaccine's safety and mobilized them to receive it.
Today, the information we consume is no longer centralized—in fact, just the opposite. "When you take that away, it's hard for people to know what to trust and what not to trust," Baum explains. We've witnessed an increase in polarization and the technology that makes it easier to give people what they want to hear, reinforcing the human tendencies to vilify the other side and reinforce our preexisting ideas. When information is engineered to further an agenda, each choice and risk calculation made while navigating the COVID-19 pandemic is deeply politicized.
This polarization maps onto a rise in socioeconomic inequality and economic uncertainty. These factors, associated with a sense of lost control, prime people to embrace misinformation, explains Baum, especially when the situation is difficult to comprehend. "The beauty of conspiratorial thinking is that it provides answers to all these questions," he says. Today's insidious fragmentation of news media accelerates the circulation of mis- and disinformation, reaching more people faster, regardless of veracity or motivation. In the case of vaccines, skepticism around their origin, safety, and motivation is intensified.
Alongside the rise in polarization, Pinker says "the emotional tone of the news has gone downward since the 1940s, and journalists consider it a professional responsibility to cover the negative." Relentless focus on everything that goes wrong further erodes public trust and paints a picture of the world getting worse. "Life saved is not a news story," says Pinker, but perhaps it should be, he continues. "If people were more aware of how much better life was generally, they might be more receptive to improvements that will continue to make life better. These improvements don't happen by themselves."
The Future Depends on Vaccine Confidence
So far, the U.S. has been unable to mitigate the catastrophic effects of the pandemic through social distancing, testing, and contact tracing. President Trump has downplayed the effects and threat of the virus, censored experts and scientists, given up on containing the spread, and mobilized his base to protest masks. The Trump Administration failed to devise a national plan, so our national plan has defaulted to hoping for the "miracle" of a vaccine. And they are "something of a miracle," Pinker says, describing vaccines as "the most benevolent invention in the history of our species." In record-breaking time, three vaccines have arrived. But their impact will be weakened unless we achieve mass vaccination. As Brunson notes, "The technology isn't the fix; it's people taking the technology."
Significant challenges remain, including facilitating widespread access and supporting on-the-ground efforts to allay concerns and build trust with specific populations with historic reasons for distrust, says Brunson. Baum predicts continuing delays as well as deaths from other causes that will be linked to the vaccine.
Still, there's every reason for hope. The new administration "has its eyes wide open to these challenges. These are the kind of problems that are amenable to policy solutions if we have the will," Baum says. He forecasts widespread vaccination by late summer and a bounce back from the economic damage, a "Good News Story" that will bolster vaccine acceptance in the future. And Pinker reminds us that science, medicine, and public health have greatly extended our lives in the last few decades, a trend that can only continue if we're willing to roll up our sleeves.

