Carl Zimmer: Genetically Editing Humans Should Not Be Our Biggest Worry
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

The award-winning New York Times science writer Carl Zimmer.
Carl Zimmer, the award-winning New York Times science writer, recently published a stellar book about human heredity called "She Has Her Mother's Laugh." Truly a magnum opus, the book delves into the cultural and scientific evolution of genetics, the field's outsize impact on society, and the new ways we might fundamentally alter our species and our planet.
"I was only prepared to write about how someday we would cross this line, and actually, we've already crossed it."
Zimmer spoke last week with editor-in-chief Kira Peikoff about the international race to edit the genes of human embryos, the biggest danger he sees for society (hint: it's not super geniuses created by CRISPR), and some outlandish possibilities for how we might reproduce in the future. This interview has been edited and condensed for clarity.
I was struck by the number of surprises you uncovered while researching human heredity, like how fetal cells can endure for a lifetime in a mother's body and brain. What was one of the biggest surprises for you?
Something that really jumped out for me was for the section on genetically modifying people. It does seem incredibly hypothetical. But then I started looking into mitochondrial replacement therapy, so-called "three parent babies." I was really surprised to discover that almost by accident, a number of genetically modified people were created this way [in the late 90s and early 2000s]. They walk among us, and they're actually fine as far as anyone can tell. I was only prepared to write about how someday we would cross this line, and actually, we've already crossed it.
And now we have the current arms race between the U.S. and China to edit diseases out of human embryos, with China being much more willing and the U.S. more reluctant. Do you think it's more important to get ahead or to proceed as ethically as possible?
I would prefer a middle road. I think that rushing into tinkering with the features of human heredity could be a disastrous mistake for a lot of reasons. On the other hand, if we completely retreat from it out of some vague fear, I think that we won't take advantage of the actual benefits that this technology might have that are totally ethically sound.
I think the United Kingdom is actually showing how you can go the middle route with mitochondrial replacement therapy. The United States has just said nope, you can't do it at all, and you have Congressmen talking about how it's just playing God or Frankenstein. And then there are countries like Mexico or the Ukraine where people are doing mitochondrial replacement therapy because there are no regulations at all. It's a wild west situation, and that's not a good idea either.
But in the UK, they said alright, well let's talk about this, let's have a debate in Parliament, and they did, and then the government came up with a well thought-through policy. They decided that they were going to allow for this, but only in places that applied for a license, and would be monitored, and would keep track of the procedure and the health of these children and actually have real data going forward. I would imagine that they're going to very soon have their first patients.
As you mentioned, one researcher recently traveled to Mexico from New York to carry out the so-called "three-parent baby" procedure in order to escape the FDA's rules. What's your take on scientists having to leave their own jurisdictions to advance their research programs under less scrutiny?
I think it's a problem when people who have a real medical need have to leave their own country to get truly effective treatment for it. On the other hand, we're seeing lots of people going abroad to countries that don't monitor all the claims that clinics are making about their treatments. So you have stem cell clinics in all sorts of places that are making all sorts of ridiculous promises. They're not delivering those results, and in some cases, they're doing harm.
"Advances in stem cell biology and reproductive biology are a much bigger challenge to our conventional ideas about heredity than CRISPR is."
It's a tricky tension for sure. Speaking of gene editing humans, you mention in the book that one of the CRISPR pioneers, Jennifer Doudna, now has recurring nightmares about Hitler. Do you think that her fears about eugenics being revived with gene editing are justified?
The word "eugenics" has a long history and it's meant different things to different people. So we have to do a better job of talking about it in the future if we really want to talk about the risks and the promises of technology like CRISPR. Eugenics in its most toxic form was an ideology that let governments, including the United States, sterilize their own citizens by the tens of thousands. Then Nazi Germany also used eugenics as a justification to exterminate many more people.
Nobody's talking about that with CRISPR. Now, are people concerned that we are going to wipe out lots of human genetic diversity with it? That would be a bad thing, but I'm skeptical that would actually ever happen. You would have to have some sort of science fiction one-world government that required every new child to be born with IVF. It's not something that keeps me up at night. Honestly, I think we have much bigger problems to worry about.
What is the biggest danger relating to genetics that we should be aware of?
Part of what made eugenics such a toxic ideology was that it was used as a justification for indifference. In other words, if there are problems in society, like a large swath of people who are living in poverty, well, there's nothing you can do about it because it must be due to genetics.
If you look at genetics as being the sole place where you can solve humanity's problems, then you're going to say well, there's no point in trying to clean up the environment or trying to improve human welfare.
A major theme in your book is that we should not narrow our focus on genes as the only type of heredity. We also may inherit some epigenetic marks, some of our mother's microbiome and mitochondria, and importantly, our culture and our environment. Why does an expanded view of heredity matter?
We should think about the world that our children are going to inherit, and their children, and their children. They're going to inherit our genes, but they're also going to inherit this planet and we're doing things that are going to have an incredibly long-lasting impact on it. I think global warming is one of the biggest. When you put carbon dioxide into the air, it stays there for a very, very long time. If we stopped emitting carbon dioxide now, the Earth would stay warm for many centuries. We should think about tinkering with the future of genetic heredity, but I think we should also be doing that with our environmental heredity and our cultural heredity.
At the end of the book, you discuss some very bizarre possibilities for inheritance that could be made possible through induced pluripotent stem cell technology and IVF -- like four-parent babies, men producing eggs, and children with 8-celled embryos as their parents. If this is where reproductive medicine is headed, how can ethics keep up?
I'm not sure actually. I think that these advances in stem cell biology and reproductive biology are a much bigger challenge to our conventional ideas about heredity than CRISPR is. With CRISPR, you might be tweaking a gene here and there, but they're still genes in an embryo which then becomes a person, who would then have children -- the process our species has been familiar with for a long time.
"We have to recognize that we need a new language that fits with the science of heredity in the 21st century."
We all assume that there's no way to find a fundamentally different way of passing down genes, but it turns out that it's not really that hard to turn a skin cell from a cheek scraping into an egg or sperm. There are some challenges that still have to be worked out to make this something that could be carried out a lot in labs, but I don't see any huge barriers to it. Ethics doesn't even have the language to discuss the possibilities. Like for example, one person producing both male and female sex cells, which are then fertilized to produce embryos so that you have a child who only has one parent. How do we even talk about that? I don't know. But that's coming up fast.
We haven't developed our language as quickly as the technology itself. So how do we move forward?
We have to recognize that we need a new language that fits with the science of heredity in the 21st century. I think one of the biggest problems we have as a society is that most of our understanding about these issues largely comes from what we learned in grade school and high school in biology class. A high school biology class, even now, gets up to Mendel and then stops. Gregor Mendel is a great place to start, but it's a really bad place to stop talking about heredity.
[Ed. Note: Zimmer's book can be purchased through your retailer of choice here.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”
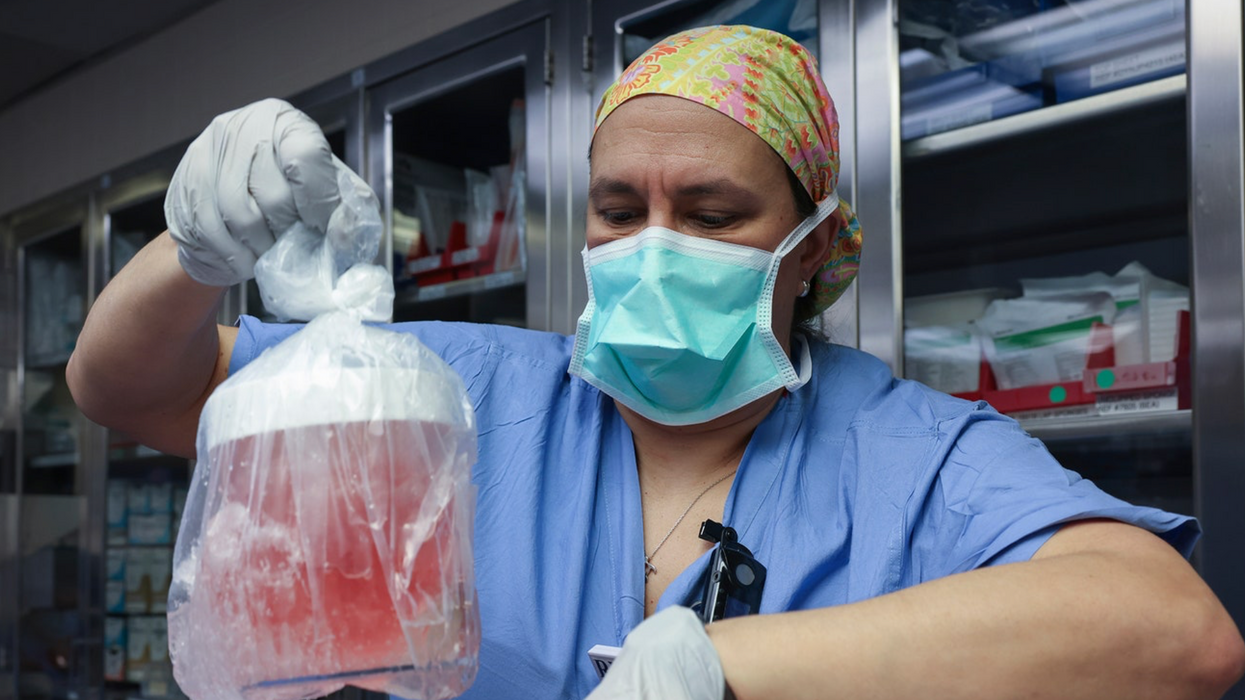
MILESTONE: Doctors have transplanted a pig organ into a human for the first time in history
A surgeon at Massachusetts General Hospital prepares a pig organ for transplant.
Surgeons at Massachusetts General Hospital made history last week when they successfully transplanted a pig kidney into a human patient for the first time ever.
The recipient was a 62-year-old man named Richard Slayman who had been living with end-stage kidney disease caused by diabetes. While Slayman had received a kidney transplant in 2018 from a human donor, his diabetes ultimately caused the kidney to fail less than five years after the transplant. Slayman had undergone dialysis ever since—a procedure that uses an artificial kidney to remove waste products from a person’s blood when the kidneys are unable to—but the dialysis frequently caused blood clots and other complications that landed him in the hospital multiple times.
As a last resort, Slayman’s kidney specialist suggested a transplant using a pig kidney provided by eGenesis, a pharmaceutical company based in Cambridge, Mass. The highly experimental surgery was made possible with the Food and Drug Administration’s “compassionate use” initiative, which allows patients with life-threatening medical conditions access to experimental treatments.
The new frontier of organ donation
Like Slayman, more than 100,000 people are currently on the national organ transplant waiting list, and roughly 17 people die every day waiting for an available organ. To make up for the shortage of human organs, scientists have been experimenting for the past several decades with using organs from animals such as pigs—a new field of medicine known as xenotransplantation. But putting an animal organ into a human body is much more complicated than it might appear, experts say.
“The human immune system reacts incredibly violently to a pig organ, much more so than a human organ,” said Dr. Joren Madsen, director of the Mass General Transplant Center. Even with immunosuppressant drugs that suppress the body’s ability to reject the transplant organ, Madsen said, a human body would reject an animal organ “within minutes.”
So scientists have had to use gene-editing technology to change the animal organs so that they would work inside a human body. The pig kidney in Slayman’s surgery, for instance, had been genetically altered using CRISPR-Cas9 technology to remove harmful pig genes and add human ones. The kidney was also edited to remove pig viruses that could potentially infect a human after transplant.
With CRISPR technology, scientists have been able to prove that interspecies organ transplants are not only possible, but may be able to successfully work long term, too. In the past several years, scientists were able to transplant a pig kidney into a monkey and have the monkey survive for more than two years. More recently, doctors have transplanted pig hearts into human beings—though each recipient of a pig heart only managed to live a couple of months after the transplant. In one of the patients, researchers noted evidence of a pig virus in the man’s heart that had not been identified before the surgery and could be a possible explanation for his heart failure.
So far, so good
Slayman and his medical team ultimately decided to pursue the surgery—and the risk paid off. When the pig organ started producing urine at the end of the four-hour surgery, the entire operating room erupted in applause.
Slayman is currently receiving an infusion of immunosuppressant drugs to prevent the kidney from being rejected, while his doctors monitor the kidney’s function with frequent ultrasounds. Slayman is reported to be “recovering well” at Massachusetts General Hospital and is expected to be discharged within the next several days.