An Investigational Drug Offers Hope to Patients with a Disabling Neuromuscular Disease

Robert Thomas was a devoted runner, gym goer, and crew member on a sailing team in San Diego when, in his 40s, he noticed that his range of movement was becoming more limited.
He thought he was just getting older, but when he was hiking an uphill trail in Lake Tahoe, he kept tripping over rocks. "I'd never had this happen before," Robert says. "I knew something was wrong but didn't know what it was."
It wasn't until age 50 when he was diagnosed with Charcot-Marie-Tooth disease. The genetic disorder damages the peripheral nerves, which connect the brain and spinal cord to the rest of the body. This network of nerves is responsible for relaying information and signals about sensation, movement, and motor coordination. Over time, the disease causes debilitating muscle weakness and the loss of limb control.
Charcot-Marie-Tooth usually presents itself in childhood or in a person's teens, but in some patients, like Robert, onset can be later in life. Symptoms may include muscle cramping, tingling, or burning. Many patients also have high foot arches or hammer toes — toes that curl from the middle joint instead of pointing forward. Those affected often have difficulty walking and may lose sensation in their lower legs, feet, hands, or forearms. One of the most common rare diseases, it affects around 130,000 people in the United States and 2.8 million worldwide.
Like many people with Charcot-Marie-Tooth, or CMT, Robert wears corrective braces on his legs to help with walking. Now 61, he can't run or sail anymore because of the disease, but he still works out regularly and can hike occasionally. CMT also affects his grip, so he has to use special straps while doing some exercises.
For the past few years, Robert has been participating in a clinical trial for an investigational CMT drug. He takes the liquid formulation every morning and evening using an oral syringe. Scientists are following patients like Robert to learn if their symptoms stabilize or improve while on the drug. Dubbed PXT300, the drug was designed by French biopharmaceutical company Pharnext and is the farthest along in development for CMT. If approved, it would be the first drug for the disease.
Currently, there's no cure for CMT, only supportive treatments like pain medication. Some individuals receive physical and occupational therapy. A drug for CMT could be a game-changer for patients whose quality of life is severely affected by the disease.
Genetic Underpinnings
CMT arises from mutations in genes that are responsible for creating and maintaining the myelin sheath — the insulating layer around nerves. Pharnext's drug is meant to treat patients with CMT1A, the most common form of the disease, which represents about half of CMT cases. Around 5% of those with CMT1A become severely disabled and end up in wheelchairs. People with CMT1A have an extra copy of the gene PMP22, which makes a protein that's needed to maintain the myelin sheath around peripheral nerves.
Typically, an individual inherits one copy of PMP22 from each parent. But a person with CMT1A receives a copy of PMP22 from one parent and two copies from a parent with the disease. This extra copy of the gene results in excess protein production, which damages the cells responsible for preserving and regenerating the myelin sheath, called Schwann cells.
The myelin sheath helps ensure that a signal from the brain gets carried to nerves in the muscles so that a part of the body can carry out a particular action or movement. This sheath is like the insulation on an electrical cord and the action is like a light bulb. If the insulation is fine, the light bulb turns on. But if the insulation is frayed, the light will flicker.
"The same happens to these patients," says David Horn Solomon, CEO of Pharnext. "The signal to their muscle is weak and flickers." Over time, their muscles become weaker and thinner.
The PMP22 gene has proven difficult to target with a drug because it's located in a protected space — the Schwann cells that make up the insulation around nerves. "There's not an easy way to tamp it down," Solomon says.
Another company, Acceleron Pharma of Cambridge, Massachusetts, was developing an injectable CMT drug meant to increase the strength of leg muscles. But the company halted development last year after the experimental drug failed in a mid-stage trial. While the drug led to a statistically significant increase in muscle volume, it didn't translate to improvements in muscle function or quality of life for trial participants.
Made by Design
Pharnext's drug, PXT3003, is a combination of three existing drugs — baclofen, a muscle relaxant; naltrexone, a drug that decreases the desire for alcohol and opioids; and sorbitol, a type of sugar alcohol.
The company designed the drug using its artificial intelligence platform, which screened 20,000 existing drugs to predict combinations that could inhibit the PMP22 gene and thereby lower protein production. The AI system narrowed the search to several hundreds of combinations and Pharnext tested around 75 of them in the lab before landing on baclofen, naltrexone, and sorbitol. Individually, the drugs don't have much effect on the PMP22 gene. But combined, they work to lower how much protein the gene makes.
"How the drug inside the cell reduces expression isn't quite clear yet," says Florian Thomas, director of the Hereditary Neuropathy Center, and founding chair and professor in the department of neurology at Hackensack University Medical Center and Hackensack Meridian School of Medicine in New Jersey (no relation to Robert Thomas, the CMT patient). "By reducing the amount of protein being produced, we hopefully can stabilize the nerves."
In rodents genetically engineered to have the PMP22 gene, the drug reduced protein levels and delayed onset of muscle weakness when given to rats. In another animal study, the drug increased the size of the myelin sheath around nerves in rats.
"Like humans with CMT, one of the problems the animals have is they can't grip things, their grip strength is poor," Solomon says. But when treated with Pharnext's drug, "the grip strength of these animals improves dramatically even over 12 weeks."
Human trials look encouraging, too. But the company ran into a manufacturing issue during a late-stage trial. The drug requires refrigeration, and as a result of temperature changes, crystals formed inside vials containing the high dose of the drug. The study was a double-blind trial, meaning neither the trial participants nor investigators were supposed to know who received the high dose of the drug, who received the low dose, and who received a placebo. In these types of studies, the placebo and experimental drug should look the same so that investigators can't tell them apart. But because only the high dose contained crystals, not the low dose or placebo, regulators said the trial data could be biased.
Pharnext is now conducting a new randomized, double-blind trial to prove that its drug works. The study is recruiting individuals aged 16 through 65 years old with mild to moderate CMT. The company hopes to show that the drug can stop patients' symptoms from worsening, or in the best case scenario, possibly even improve them. The company doesn't think the drug will be able to help people with severe forms of the disease.
"In neurologic disease, you're looking for plasticity, where there's still the possibility of stabilization or reversal," Solomon says. Plasticity refers to the ability of the nervous system to change and adapt in response to stimuli.
Preventing Disability
Allison Moore, a CMT patient and founder and CEO of the Hereditary Neuropathy Foundation, has been following drug development for CMT since she founded the organization in 2001. She says many investigational drugs haven't moved forward because they've shown little success in animals. The fact that Pharnext's drug has made it to a late-stage human trial is promising, she says.
"It's really exciting," Moore says. "There's a chance that if you take the drug early before you're very severe, you'll end up not developing the disease to a level that's super disabling."
CMT has damaged Moore's peroneal nerve, a main nerve in the foot. As a result, she has foot drop, the inability to lift the front part of her foot, and needs to wear leg braces to help her walk. "The idea that you could take this early on and that it could stop progression, that's the hope that we have."
Thomas, the neurologist, says a drug doesn't have to be a cure to have a significant impact on patients. "If I have a CMT patient who's 50 years old, that patient will be more disabled by age 60," he says. "If I can treat that person with a drug, and that person is just as disabled at age 60 as they were at age 50, that's transformative in my mind."
While Robert Thomas says he hasn't noticed a dramatic improvement since he's been on the drug, he does think it's helping. Robert is now in an open-label study, which means he and his health provider are aware that he's receiving the drug.
When the COVID-19 pandemic hit, manufacturing and supply chain disruptions meant that Robert was without the trial drug for two months. When his medication ran out, his legs felt unstable again and walking was harder. "There was a clear distinction between being on and off that medication," he says.
Pharnext's current trial will take about a year and a half to complete. After that, the FDA will decide on whether to approve the drug for CMT patients.
As scientists learn more about the PMP22 gene and the more than 100 other genes that when mutated cause CMT, more precise treatments could be possible. For instance, scientists have used the gene-editing tool CRISPR to correct a CMT-causing mutation in human cells in the lab. The results were published August 16 in the journal Frontiers in Cell and Developmental Biology.
Pharnext is also interested in pursuing genetic treatments for CMT, but in the meantime, repurposed drugs may be the best shot at helping patients until more advanced treatments are available.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
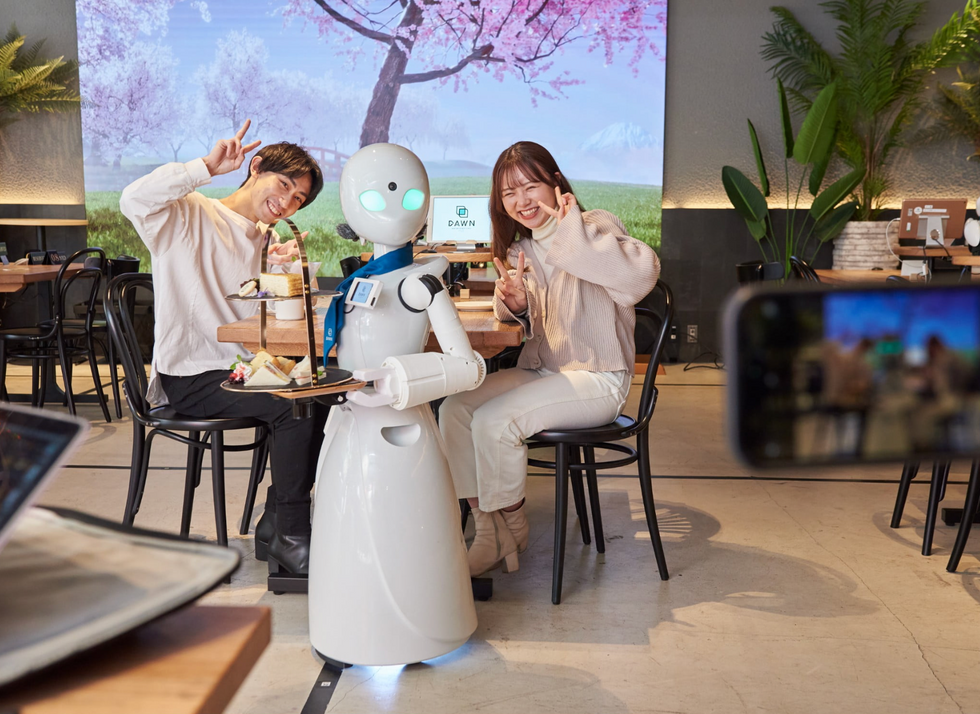
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
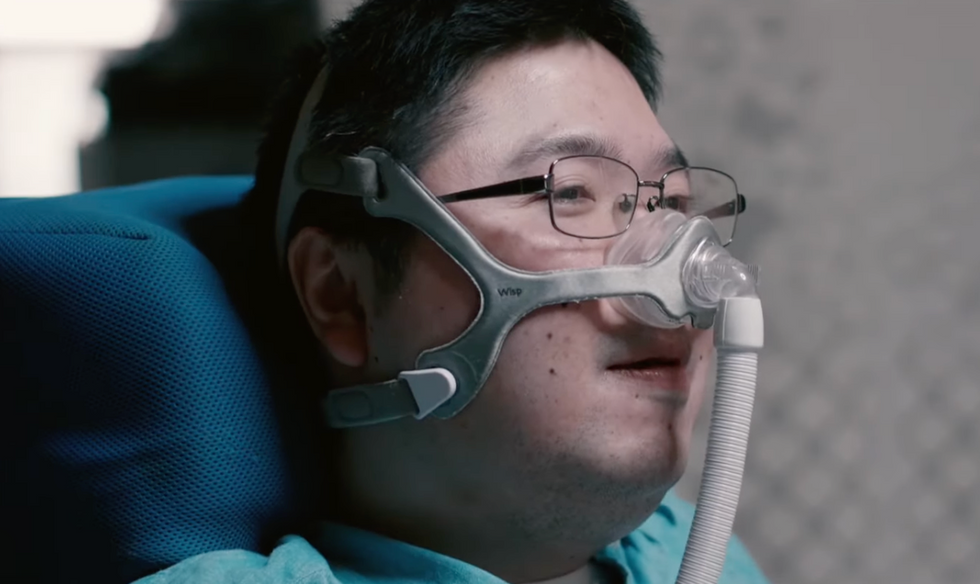
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

