Researchers advance drugs that treat pain without addiction
New therapies are using creative approaches that target the body’s sensory neurons, which send pain signals to the brain.
Opioids are one of the most common ways to treat pain. They can be effective but are also highly addictive, an issue that has fueled the ongoing opioid crisis. In 2020, an estimated 2.3 million Americans were dependent on prescription opioids.
Opioids bind to receptors at the end of nerve cells in the brain and body to prevent pain signals. In the process, they trigger endorphins, so the brain constantly craves more. There is a huge risk of addiction in patients using opioids for chronic long-term pain. Even patients using the drugs for acute short-term pain can become dependent on them.
Scientists have been looking for non-addictive drugs to target pain for over 30 years, but their attempts have been largely ineffective. “We desperately need alternatives for pain management,” says Stephen E. Nadeau, a professor of neurology at the University of Florida.
A “dimmer switch” for pain
Paul Blum is a professor of biological sciences at the University of Nebraska. He and his team at Neurocarrus have created a drug called N-001 for acute short-term pain. N-001 is made up of specially engineered bacterial proteins that target the body’s sensory neurons, which send pain signals to the brain. The proteins in N-001 turn down pain signals, but they’re too large to cross the blood-brain barrier, so they don’t trigger the release of endorphins. There is no chance of addiction.
When sensory neurons detect pain, they become overactive and send pain signals to the brain. “We wanted a way to tone down sensory neurons but not turn them off completely,” Blum reveals. The proteins in N-001 act “like a dimmer switch, and that's key because pain is sensation overstimulated.”
Blum spent six years developing the drug. He finally managed to identify two proteins that form what’s called a C2C complex that changes the structure of a subunit of axons, the parts of neurons that transmit electrical signals of pain. Changing the structure reduces pain signaling.
“It will be a long path to get to a successful clinical trial in humans," says Stephen E. Nadeau, professor of neurology at the University of Florida. "But it presents a very novel approach to pain reduction.”
Blum is currently focusing on pain after knee and ankle surgery. Typically, patients are treated with anesthetics for a short time after surgery. But anesthetics usually only last for 4 to 6 hours, and long-term use is toxic. For some, the pain subsides. Others continue to suffer after the anesthetics have worn off and start taking opioids.
N-001 numbs sensation. It lasts for up to 7 days, much longer than any anesthetic. “Our goal is to prolong the time before patients have to start opioids,” Blum says. “The hope is that they can switch from an anesthetic to our drug and thereby decrease the likelihood they're going to take the opioid in the first place.”
Their latest animal trial showed promising results. In mice, N-001 reduced pain-like behaviour by 90 percent compared to the control group. One dose became effective in two hours and lasted a week. A high dose had pain-relieving effects similar to an opioid.
Professor Stephen P. Cohen, director of pain operations at John Hopkins, believes the Neurocarrus approach has potential but highlights the need to go beyond animal testing. “While I think it's promising, it's an uphill battle,” he says. “They have shown some efficacy comparable to opioids, but animal studies don't translate well to people.”
Nadeau, the University of Florida neurologist, agrees. “It will be a long path to get to a successful clinical trial in humans. But it presents a very novel approach to pain reduction.”
Blum is now awaiting approval for phase I clinical trials for acute pain. He also hopes to start testing the drug's effect on chronic pain.
Learning from people who feel no pain
Like Blum, a pharmaceutical company called Vertex is focusing on treating acute pain after surgery. But they’re doing this in a different way, by targeting a sodium channel that plays a critical role in transmitting pain signals.
In 2004, Stephen Waxman, a neurology professor at Yale, led a search for genetic pain anomalies and found that biologically related people who felt no pain despite fractures, burns and even childbirth had mutations in the Nav1.7 sodium channel. Further studies in other families who experienced no pain showed similar mutations in the Nav1.8 sodium channel.
Scientists set out to modify these channels. Many unsuccessful efforts followed, but Vertex has now developed VX-548, a medicine to inhibit Nav1.8. Typically, sodium ions flow through sodium channels to generate rapid changes in voltage which create electrical pulses. When pain is detected, these pulses in the Nav1.8 channel transmit pain signals. VX-548 uses small molecules to inhibit the channel from opening. This blocks the flow of sodium ions and the pain signal. Because Nav1.8 operates only in peripheral nerves, located outside the brain, VX-548 can relieve pain without any risk of addiction.
"Frankly we need drugs for chronic pain more than acute pain," says Waxman.
The team just finished phase II clinical trials for patients following abdominoplasty surgery and bunionectomy surgery.
After abdominoplasty surgery, 76 patients were treated with a high dose of VX-548. Researchers then measured its effectiveness in reducing pain over 48 hours, using the SPID48 scale, in which higher scores are desirable. The score for Vertex’s drug was 110.5 compared to 72.7 in the placebo group, whereas the score for patients taking an opioid was 85.2. The study involving bunionectomy surgery showed positive results as well.
Waxman, who has been at the forefront of studies into Nav1.7 and Nav1.8, believes that Vertex's results are promising, though he highlights the need for further clinical trials.
“Blocking Nav1.8 is an attractive target,” he says. “[Vertex is] studying pain that is relatively simple and uniform, and that's key to having a drug trial that is informative. But the study needs to be replicated and frankly we need drugs for chronic pain more than acute pain. If this is borne out by additional studies, it's one important step in a journey.”
Vertex will be launching phase III trials later this year.
Finding just the right amount of Nerve Growth Factor
Whereas Neurocarrus and Vertex are targeting short-term pain, a company called Levicept is concentrating on relieving chronic osteoarthritis pain. Around 32.5 million Americans suffer from osteoarthritis. Patients commonly take NSAIDs, or non-steroidal anti-inflammatory drugs, but they cannot be taken long-term. Some take opioids but they aren't very effective.
Levicept’s drug, Levi-04, is designed to modify a signaling pathway associated with pain. Nerve Growth Factor (NGF) is a neurotrophin: it’s involved in nerve growth and function. NGF signals by attaching to receptors. In pain there are excess neurotrophins attaching to receptors and activating pain signals.
“What Levi-04 does is it returns the natural equilibrium of neurotrophins,” says Simon Westbrook, the CEO and founder of Levicept. It stabilizes excess neurotrophins so that the NGF pathway does not signal pain. Levi-04 isn't addictive since it works within joints and in nerves outside the brain.
Westbrook was initially involved in creating an anti-NGF molecule for Pfizer called Tanezumab. At first, Tanezumab seemed effective in clinical trials and other companies even started developing their own versions. However, a problem emerged. Tanezumab caused rapidly progressive osteoarthritis, or RPOA, in some patients because it completely removed NGF from the system. NGF is not just involved in pain signalling, it’s also involved in bone growth and maintenance.
Levicept has found a way to modify the NGF pathway without completely removing NGF. They have now finished a small-scale phase I trial mainly designed to test safety rather than efficacy. “We demonstrated that Levi-04 is safe and that it bound to its target, NGF,” says Westbrook. It has not caused RPOA.
Professor Philip Conaghan, director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine, believes that Levi-04 has potential but urges the need for caution. “At this early stage of development, their molecule looks promising for osteoarthritis pain,” he says. “They will have to watch out for RPOA which is a potential problem.”
Westbrook starts phase II trials with 500 patients this summer to check for potential side effects and test the drug’s efficacy.
There is a real push to find an effective alternative to opioids. “We have a lot of work to do,” says Professor Waxman. “But I am confident that we will be able to develop new, much more effective pain therapies.”
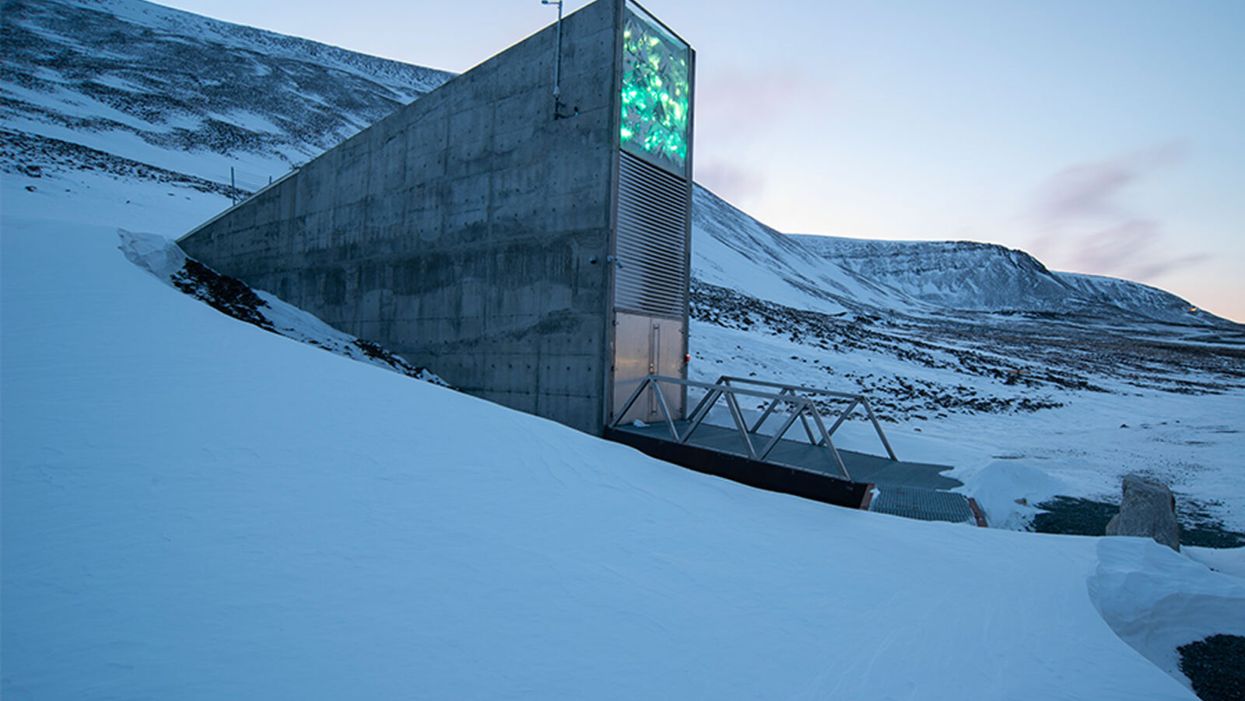
Over 1 Million Seeds Are Buried Near the North Pole to Back Up the World’s Crops
The Svalbard Global Seed Vault preserves more than one million of the world's seeds deep inside Platäfjellet Mountain.
The impressive structure protrudes from the side of a snowy mountain on the Svalbard Archipelago, a cluster of islands about halfway between Norway and the North Pole.
"Before, we trusted the permafrost. We do not trust the permafrost anymore."
Art installations on the building's rooftop and front façade glimmer like diamonds in the polar night, but it is what lies buried deep inside the frozen rock, 475 feet from the building's entrance, that is most precious. Here, in the Svalbard Global Seed Vault, are backup copies of more than a million of the world's agricultural seeds.

Inside the vault, seed boxes from many gene banks and many countries. "The seeds don't know national boundaries," says Kent Nnadozie, the UN's Secretary of the International Treaty on Plant Genetic Resources for Food and Agriculture.
(Photo credit: Svalbard Global Seed Vault/Riccardo Gangale)
The Svalbard vault -- which has been called the Doomsday Vault, or a Noah's Ark for seeds -- preserves the genetic materials of more than 6000 crop species and their wild relatives, including many of the varieties within those species. Svalbard's collection represents all the traits that will enable the plants that feed the world to adapt – with the help of farmers and plant breeders – to rapidly changing climactic conditions, including rising temperatures, more intense drought, and increasing soil salinity. "We save these seeds because we want to ensure food security for future generations," says Grethe Helene Evjen, Senior Advisor at the Norwegian Ministry of Agriculture and Food .
A recent study in the journal Nature predicted that global warming could cause catastrophic losses of biodiversity in regions across the globe throughout this century. Yet global warming also threatens the permafrost that surrounds the seed vault, the very thing that was once considered a failsafe means of keeping these seeds frozen and safeguarding the diversity of our crops. In fact, record temperatures in Svalbard a few years ago – and a significant breach of water into the access tunnel to the vault -- prompted the Norwegian government to invest $20 million euros on improvements at the facility to further secure the genetic resources locked inside. The hope: that technology can work in concert with nature's freezer to keep the world's seeds viable.
"Before, we trusted the permafrost," says Hege Njaa Aschim, a spokesperson for Statsbygg, the government agency that recently completed the upgrades at the seed vault. "We do not trust the permafrost anymore."
The Apex of the Global Conservation System
More than 1700 genebanks around the globe preserve the diverse seed varieties from their regions. They range from small community seed banks in developing countries, where small farmers save and trade their seeds with growers in nearby villages, to specialized university collections, to national and international genetic resource repositories. But many of these facilities are vulnerable to war, natural disasters, or even lack of funding.
"If anything should happen to the resources in a regular genebank, Svalbard is the backup – it's essentially the apex of the global conservation system," says Kent Nnadozie, Secretary of the International Treaty on Plant Genetic Resources for Food and Agriculture at the United Nations, who likens the Global Vault to the Central Reserve Bank. "You have regular banks that do active trading, but the Central Bank is the final reserve where the banks store their gold deposits."
Similarly, farmers deposit their seeds in regional genebanks, and also look to these banks for new varieties to help their crops adapt to, say, increasing temperatures, or resist intrusive pests. Regional banks, in turn, store duplicates from their collections at Svalbard. These seeds remain the sovereign property of the country or institution depositing them; only they can "make a withdrawal."
The Global Vault has already proven invaluable: The International Centre for Agricultural Research in the Dry Areas (ICARDA), formerly located outside of Aleppo, Syria, held more than 140,000 seed samples, including plants that were extinct in their natural habitats, before the Syrian Crisis in 2012. Fortunately, they had managed to back up most of their seed samples at Svalbard before they were forced to relocate to Lebanon and Morocco. In 2017, ICARDA became the first – and only – organization to withdraw their stored seeds. They have now regenerated almost all of the samples at their new locations and recently redeposited new seeds for safekeeping at Svalbard.
Rapid Global Warming Threatens Permafrost
The Global Vault, a joint venture between the Norwegian government, the Crop Trust and the Nordic Genetic Resource Centre (NordGen) that started operating in 2008, was sited in Svalbard in part because of its remote yet accessible location: Svalbard is the northernmost inhabited spot on Earth with an airport. But experts also thought it a failsafe choice for long-term seed storage because its permafrost would offer natural freezing – even if cooling systems were to fail. No one imagined that the permafrost could fail.
"We've had record temperatures in the region recently, and there are a lot of signs that global warming is happening faster at the extreme latitudes," says Geoff Hawtin, a world-renowned authority in plant conservation, who is the founding director of -- and now advisor to -- the Crop Trust. "Svalbard is still arguably one of the safest places for the seeds from a temperature point of view, but it's actually not going to be as cold as we thought 20 years ago."
A recent report by the Norwegian Centre for Climate Services predicted that Svalbard could become 50 degrees Fahrenheit warmer by the year 2100. And data from the Norwegian government's environmental monitoring system in Svalbard shows that the permafrost is already thawing: The "active layer," that is, the layer of surface soil that seasonally thaws, has become 25-30 cm thicker since 1998.
Among the 35 depositors were several bringing their seeds to Svalbard for the first time, including the Cherokee Nation, which deposited nine heirloom seed varieties that predate European colonization.
Though the permafrost surrounding the seed vault chambers, which are situated well below the active layer, is still intact, the permafrost around the access tunnel never re-established as expected after construction of the Global Vault twelve years ago. As a result, when Svalbard saw record high temperatures and unprecedented rainfall in 2016, about 164 feet of rainwater and snowmelt leaked into the tunnel, turning it into a skating rink and spurring authorities to take what they called a "better safe than sorry approach." They invested in major upgrades to the facility. "The seeds in the vault were never threatened," says Aschim, "but technology has become more important at Svalbard."
Technology Gives Nature a Boost
For now, the permafrost deep inside the mountain still keeps the temperature in the vault down to about -25°F. The cooling systems then give nature a mechanical boost to keep the seed vault chilled even further, to about -64°F, the optimal temperature for conserving seeds. In addition to upgrading to a more effective and sustainable cooling system that runs on CO2, the Norwegian government added backup generators, removed heat-generating electrical equipment from inside the facility to an outside building, installed a thick, watertight door to the vault, and replaced the corrugated steel access tunnel with a cement tunnel that uses the same waterproofing technology as the North Sea oil platforms.
To re-establish the permafrost around the tunnel, they layered cooling pipes with frozen soil around the concrete tunnel, covered the frozen soil with a cooling mat, and topped the cooling mat with the original permafrost soil. They also added drainage ditches on the mountainside to divert meltwater away from the tunnel as the climate gets warmer and wetter.
New Deposits to the Global Vault
The day before COVID-19 arrived in Norway, on February 25th, Prime Minister Erna Solberg hosted the biggest seed-depositing event in the vault's history in honor of the new and improved vault. As snow fell on Svalbard, depositors from almost every continent traveled the windy road from Longyearbyen up Platåfjellet Mountain and braved frigid -8°F weather to celebrate the massive technical upgrades to the facility – and to hand over their seeds.
Among the 35 depositors were several bringing their seeds to Svalbard for the first time, including the Cherokee Nation, which deposited nine heirloom seed varieties that predate European colonization, and Israel's University of Haifa, whose deposit included multiple genotypes of wild emmer wheat, an ancient relative of the modern domesticated crop. The storage boxes carried ceremoniously over the threshold that day contained more than 65,000 new seed samples, bringing the total to more than a million, and almost filling the first of three seed chambers in the vault. (The Global Vault can store up to 4.5 million seed samples.)
"Svalbard's samples contain all the possibilities, all the options for the future of our agricultural crops – it's how crops are going to adapt," says Cary Fowler, former executive director of the Crop Trust, who was instrumental in establishing the Global Vault. "If our crops don't adapt to climate change, then neither will we." Dr. Fowler says he is confident that with the recent improvements in the vault, the seeds are going to remain viable for a very long time.
"It's sometimes tempting to get distracted by the romanticism of a seed vault inside a mountain near the North Pole – it's a little bit James Bondish," muses Dr. Fowler. "But the reality is we've essentially put an end to the extinction of more than a million samples of biodiversity forever."
From Crap to Cure: The Story of Fecal Transplants
Meg Newman, who suffered from C. difficile, underwent a fecal transplant that helped restore her to health.
C. difficile had Meg Newman's number; it had struck her 18 different times beginning in 1985. The bacterial infection takes over the gut bringing explosive diarrhea, dehydration, weight loss, and at its worst depletes blood platelets. It causes nearly 30,000 deaths each year in the U.S. alone.
"I was one sick puppy as that point and literally three days after the transplant I was doing pretty well, day four even better."
Meg knew these statistics not just from personal experience but also because she was a doctor at San Francisco General Hospital. Antibiotics had sometimes helped to treat the infection, but it never quite seemed to go away. Now, "It felt like part of my colon was sort of sliding off as I had the bowel movement." On her worst day she counted 33 bowel movements. It was 2005 and she knew she was at the end of her rope.
Medical training had taught Meg to look at the data. So when antibiotics failed, she searched the literature for other options. One was a seemingly off-the-wall treatment called fecal transplants, which essentially gives poop from a healthy person to one who is sick.
Its roots stretch back to "yellow soup" used to treat dysentery in China nearly two thousand years ago, in which ancient Chinese treaters would combine stool with liquid, mash it up, and administer it. The approach also is commonly used in veterinary medicine today. However, there were only about three papers on its use in humans in the medical literature at that time, she recalls. Still, the logic of the intervention appealed to her.
The gut microbiome as a concept and even a word were not widely known fifteen years ago. But the idea that the microbial community in her gut was in disarray, and a transplant of organisms from a healthy gut might help restore a more normal ecology made sense. And besides, the failure of standard medicine left her few options.
Meg spoke with a colleague, gastroenterologist Neil Stollman, about a possible fecal microbial transplant (FMT). Only a handful of doctors in the U.S. had ever done the procedure; Stollman had tried it just once before. After conversation with Newman, he agreed to do it.
They decided on Meg's partner Sherry as the donor. "I try very hard to use intimate sexual partners as the donor," explains Stollman. The reason is to reduce disease risk: "The logic there is pretty straightforward. If you have unprotected sex with this individual, in a monogamous way for a period of time, you have assumed pretty much any infectious risk," like hepatitis, HIV, and syphilis, he says. Other donors would be screened using the same criteria used to screen blood donations, plus screening for parasites that can live in stool but not blood.
The procedure
Martini aficionados fall into two camps, fans of shaken or stirred. In the early days the options for producing of fecal transplants were a blender or hand shaken. Stollman took the hands-on approach, mixing Sherry's fecal donation with saline to create "a milkshake in essence." He filtered it several times through gauze to get out the lumps.
Then he inserted a colonoscope, a long flexible tube, through the anus into Meg's colon. Generally a camera goes through the tube to look for polyps and cancers, while other tools can snip off polyps and retrieve tissue samples. Today he used it to insert the fecal "milkshake" as high up the colon as he could go. Imodium and bed rest were the final pieces. It works about 90 percent of the time today.
Meg went home with fingers crossed. "And within about two weeks things just slowed down; the diarrhea just stopped. I felt better so my appetite was better." The tide had turned, though it would take months to slowly repair the toll taken on her body from disease and antibiotics.
Then in 2011 another serious medical challenge required heavy use of antibiotics and Meg's C. difficile came roaring back; she needed a second FMT. Sherry had a bad sinus infection and had been on antibiotics, so that ruled her out as a donor. Red, Meg's godson, volunteered. He was twenty-one and had little or no exposure to antibiotics, which can harm friendly bacteria living in the gut.
"I was one sick puppy as that point," Meg recalls, "and literally three days after the transplant [from Red] I was doing pretty well, day four even better. It was unbelievable." It illustrated that donors are not the same, and that while an intimate partner may be the safest option, it also may not be the most efficacious donation in terms of providing missing parts of the microbial ecosystem.
Going mainstream
By then, FMTs were starting to come out of the shadows as more than just a medical oddity. One gigantic milestone in changing perceptions was a Dutch study on using the procedure to treat C. difficile that was published in January 2013 in the New England Journal of Medicine. "That was the first trial where people said, look this isn't voodoo. This wasn't made up; it really worked," says Colleen Kelly, another pioneer in using FMTs to treat C. difficile and a researcher at Brown University. A single dose was successful more than 80 percent of the time in resolving disease in patients who had failed multiple regimens of antibiotics.
Charlatans pounced on the growing interest in the microbiome, promoting FMT as a cure for all sorts of ailments for which there was no scientific evidence. The FDA stepped in, announcing it would regulate the procedure as a drug, and essentially banned use of FMTs until it wrote regulations. But the outcry from physicians and patients was so great it was forced to retreat and has allowed continued use in treating C. difficile albeit on an interim regulatory basis that has continued since 2013.
Another major change was the emergence of stool banks, modeled on blood banks. The most widely know is OpenBiome, established in 2012 as a nonprofit by young researchers at Harvard and MIT. It aimed to standardize donation of stool and screening for disease, and package them in frozen form for colonoscopic delivery, or pill form. It greatly simplified the process and more doctors became willing to use FMTs to treat C. difficile. Today, some gastroenterologists specialize in administering the transplants as a feature of their practice.
To be sure, there have been some setbacks, including a transplant between family members where the recipient became obese, likely in part because of bacteria in the material she received. The same thing has occurred in studies in mice. And last year, an elderly man died from a toxic strain of E. coli that was in material provided by a stool bank. That caused the FDA to add that pathogen to the list of those one must screen for in products designed for use as fecal transplants.
Cost remains an issue. OpenBiome charges $1500-$2000 per transplant dose, depending on whether a frozen or pill form of the product is used. And that is likely to go up as the FDA increases the number of diseases that must be screened for, such as the virus that causes COVID-19, which is present in feces and likely can be transmitted through feces. Most insurance companies do not cover FMTs because no product has been formally approved for use by the FDA.
One of the greatest treatment challenges is making the correct diagnosis, says physician Robin Patel, who initially treated patients at the Mayo Clinic in Rochester, Minnesota but now spends most of her time there developing new diagnostics. Many things can cause diarrhea and the simple presence of the organism does not mean that C. difficile is causing it. In addition, many people are colonized with the bug but never develop symptoms of the disease.
Patel used the expensive tool of whole genome sequencing to look in great detail at C. difficile in patients who were treated with antibiotics for the infection and had recurrent diarrhea. "Some of them, as you might predict, were getting their symptoms with the same exact strain [of C. difficile] but others were not, it was a different strain," suggesting that they had been reinfected.
If it is a different strain, you might want to try antibiotics, she says, but if the same strain is present, then you might want to try a different approach such as FMT. Whole genome sequencing is still too slow and expensive to use in regularly treating patients today, but Patel hopes to develop a rapid, cost-effective test to help doctors make those types of decisions.
Biotech companies are trying to develop alternatives to poop as a source for transplant to treat C. difficile. They are picking and choosing different bacteria that they can grow and then combine into a product, to varying degrees of success, but none have yet crossed the finish line of FDA approval.
"I think [the future of FMTs] is going to be targeted, even custom-made."
The FDA would like such a product because it is used to dealing with small molecule drugs that are standardized and produced in batches. Companies are pursing it because, as Kelly says, they are like sharks "smelling money in the water." Approval of such a product might cause the FDA to shut down existing stool banks that now exist in a regulatory limbo, leaving the company with a monopoly of exclusive rights to the treatment.
Back when Meg received her first fecal transplant, the procedure was so obscure that the guidelines for treating C. difficile put out by the American College of Gastroenterology didn't even mention FMT. The procedure crept into the 2013 revision of those guidelines as a treatment of last resort. Guidance under review for release later this year or early next year will ease use further but stop short of making it a first option.
Stollman imagines a future holy grail in treating C. difficile: "You give me a stool specimen and I run it through a scanner that tells me you have too much of this and too little of that. I have a sense of what normal [microbial composition of the gut] should be and add some of this and subtract some of that. Maybe I even give you some antibiotics to get rid of some of the bad guys, give you some probiotics. I think it is going to be targeted, even custom-made."
His complete vision for treating C. difficile won't arrive tomorrow, but given how rapidly FMTs have become part of medicine, it is likely to arrive in pieces and more quickly than one might think.
About five years ago Meg discovered she had an antibody deficiency that contributed to her health problems, including vulnerability to C. difficile. She began supplementation with immunoglobulin and "that has made a huge difference in my health. It is just unbelievable how much better I am." She is grateful that fecal transplants gave her the time to figure that out. She believes "there's every reason to consider it [FMT] as a first-line treatment and do the studies, ASAP."