Researchers advance drugs that treat pain without addiction
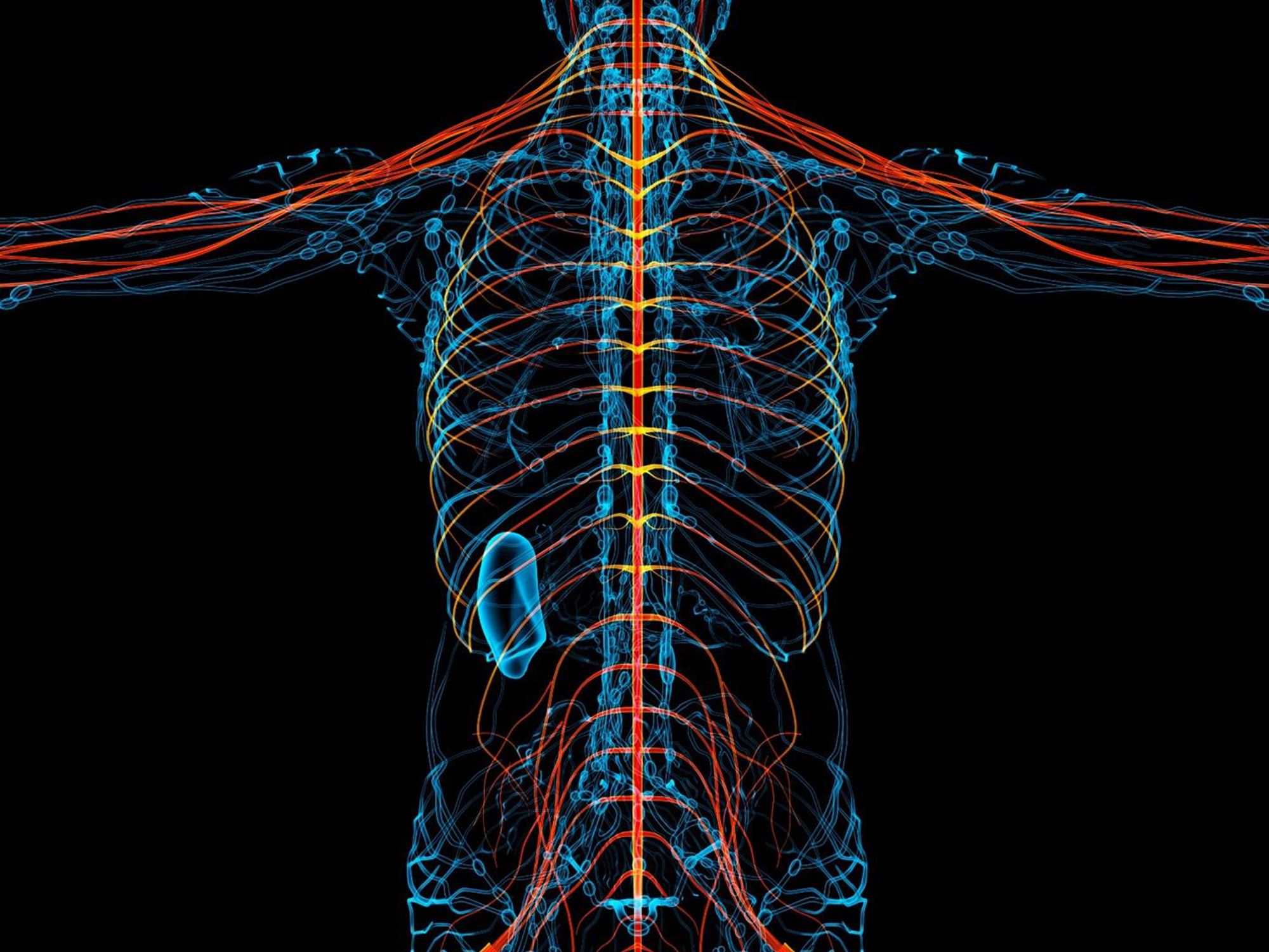
New therapies are using creative approaches that target the body’s sensory neurons, which send pain signals to the brain.
Opioids are one of the most common ways to treat pain. They can be effective but are also highly addictive, an issue that has fueled the ongoing opioid crisis. In 2020, an estimated 2.3 million Americans were dependent on prescription opioids.
Opioids bind to receptors at the end of nerve cells in the brain and body to prevent pain signals. In the process, they trigger endorphins, so the brain constantly craves more. There is a huge risk of addiction in patients using opioids for chronic long-term pain. Even patients using the drugs for acute short-term pain can become dependent on them.
Scientists have been looking for non-addictive drugs to target pain for over 30 years, but their attempts have been largely ineffective. “We desperately need alternatives for pain management,” says Stephen E. Nadeau, a professor of neurology at the University of Florida.
A “dimmer switch” for pain
Paul Blum is a professor of biological sciences at the University of Nebraska. He and his team at Neurocarrus have created a drug called N-001 for acute short-term pain. N-001 is made up of specially engineered bacterial proteins that target the body’s sensory neurons, which send pain signals to the brain. The proteins in N-001 turn down pain signals, but they’re too large to cross the blood-brain barrier, so they don’t trigger the release of endorphins. There is no chance of addiction.
When sensory neurons detect pain, they become overactive and send pain signals to the brain. “We wanted a way to tone down sensory neurons but not turn them off completely,” Blum reveals. The proteins in N-001 act “like a dimmer switch, and that's key because pain is sensation overstimulated.”
Blum spent six years developing the drug. He finally managed to identify two proteins that form what’s called a C2C complex that changes the structure of a subunit of axons, the parts of neurons that transmit electrical signals of pain. Changing the structure reduces pain signaling.
“It will be a long path to get to a successful clinical trial in humans," says Stephen E. Nadeau, professor of neurology at the University of Florida. "But it presents a very novel approach to pain reduction.”
Blum is currently focusing on pain after knee and ankle surgery. Typically, patients are treated with anesthetics for a short time after surgery. But anesthetics usually only last for 4 to 6 hours, and long-term use is toxic. For some, the pain subsides. Others continue to suffer after the anesthetics have worn off and start taking opioids.
N-001 numbs sensation. It lasts for up to 7 days, much longer than any anesthetic. “Our goal is to prolong the time before patients have to start opioids,” Blum says. “The hope is that they can switch from an anesthetic to our drug and thereby decrease the likelihood they're going to take the opioid in the first place.”
Their latest animal trial showed promising results. In mice, N-001 reduced pain-like behaviour by 90 percent compared to the control group. One dose became effective in two hours and lasted a week. A high dose had pain-relieving effects similar to an opioid.
Professor Stephen P. Cohen, director of pain operations at John Hopkins, believes the Neurocarrus approach has potential but highlights the need to go beyond animal testing. “While I think it's promising, it's an uphill battle,” he says. “They have shown some efficacy comparable to opioids, but animal studies don't translate well to people.”
Nadeau, the University of Florida neurologist, agrees. “It will be a long path to get to a successful clinical trial in humans. But it presents a very novel approach to pain reduction.”
Blum is now awaiting approval for phase I clinical trials for acute pain. He also hopes to start testing the drug's effect on chronic pain.
Learning from people who feel no pain
Like Blum, a pharmaceutical company called Vertex is focusing on treating acute pain after surgery. But they’re doing this in a different way, by targeting a sodium channel that plays a critical role in transmitting pain signals.
In 2004, Stephen Waxman, a neurology professor at Yale, led a search for genetic pain anomalies and found that biologically related people who felt no pain despite fractures, burns and even childbirth had mutations in the Nav1.7 sodium channel. Further studies in other families who experienced no pain showed similar mutations in the Nav1.8 sodium channel.
Scientists set out to modify these channels. Many unsuccessful efforts followed, but Vertex has now developed VX-548, a medicine to inhibit Nav1.8. Typically, sodium ions flow through sodium channels to generate rapid changes in voltage which create electrical pulses. When pain is detected, these pulses in the Nav1.8 channel transmit pain signals. VX-548 uses small molecules to inhibit the channel from opening. This blocks the flow of sodium ions and the pain signal. Because Nav1.8 operates only in peripheral nerves, located outside the brain, VX-548 can relieve pain without any risk of addiction.
"Frankly we need drugs for chronic pain more than acute pain," says Waxman.
The team just finished phase II clinical trials for patients following abdominoplasty surgery and bunionectomy surgery.
After abdominoplasty surgery, 76 patients were treated with a high dose of VX-548. Researchers then measured its effectiveness in reducing pain over 48 hours, using the SPID48 scale, in which higher scores are desirable. The score for Vertex’s drug was 110.5 compared to 72.7 in the placebo group, whereas the score for patients taking an opioid was 85.2. The study involving bunionectomy surgery showed positive results as well.
Waxman, who has been at the forefront of studies into Nav1.7 and Nav1.8, believes that Vertex's results are promising, though he highlights the need for further clinical trials.
“Blocking Nav1.8 is an attractive target,” he says. “[Vertex is] studying pain that is relatively simple and uniform, and that's key to having a drug trial that is informative. But the study needs to be replicated and frankly we need drugs for chronic pain more than acute pain. If this is borne out by additional studies, it's one important step in a journey.”
Vertex will be launching phase III trials later this year.
Finding just the right amount of Nerve Growth Factor
Whereas Neurocarrus and Vertex are targeting short-term pain, a company called Levicept is concentrating on relieving chronic osteoarthritis pain. Around 32.5 million Americans suffer from osteoarthritis. Patients commonly take NSAIDs, or non-steroidal anti-inflammatory drugs, but they cannot be taken long-term. Some take opioids but they aren't very effective.
Levicept’s drug, Levi-04, is designed to modify a signaling pathway associated with pain. Nerve Growth Factor (NGF) is a neurotrophin: it’s involved in nerve growth and function. NGF signals by attaching to receptors. In pain there are excess neurotrophins attaching to receptors and activating pain signals.
“What Levi-04 does is it returns the natural equilibrium of neurotrophins,” says Simon Westbrook, the CEO and founder of Levicept. It stabilizes excess neurotrophins so that the NGF pathway does not signal pain. Levi-04 isn't addictive since it works within joints and in nerves outside the brain.
Westbrook was initially involved in creating an anti-NGF molecule for Pfizer called Tanezumab. At first, Tanezumab seemed effective in clinical trials and other companies even started developing their own versions. However, a problem emerged. Tanezumab caused rapidly progressive osteoarthritis, or RPOA, in some patients because it completely removed NGF from the system. NGF is not just involved in pain signalling, it’s also involved in bone growth and maintenance.
Levicept has found a way to modify the NGF pathway without completely removing NGF. They have now finished a small-scale phase I trial mainly designed to test safety rather than efficacy. “We demonstrated that Levi-04 is safe and that it bound to its target, NGF,” says Westbrook. It has not caused RPOA.
Professor Philip Conaghan, director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine, believes that Levi-04 has potential but urges the need for caution. “At this early stage of development, their molecule looks promising for osteoarthritis pain,” he says. “They will have to watch out for RPOA which is a potential problem.”
Westbrook starts phase II trials with 500 patients this summer to check for potential side effects and test the drug’s efficacy.
There is a real push to find an effective alternative to opioids. “We have a lot of work to do,” says Professor Waxman. “But I am confident that we will be able to develop new, much more effective pain therapies.”
Dr. Steffanie Strathdee, an infectious disease epidemiologist, saved her husband Tom from a superbug infection by mobilizing scientists around the world to help him access experimental phage therapy.
[Editor's Note: This is the fifth episode in our Moonshot series, which explores cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
The latest tools, like whole genome sequencing, are allowing food outbreaks to be identified and stopped more quickly.
With the pandemic at the forefront of everyone's minds, many people have wondered if food could be a source of coronavirus transmission. Luckily, that "seems unlikely," according to the CDC, but foodborne illnesses do still sicken a whopping 48 million people per year.
Whole genome sequencing is like "going from an eight-bit image—maybe like what you would see in Minecraft—to a high definition image."
In normal times, when there isn't a historic global health crisis infecting millions and affecting the lives of billions, foodborne outbreaks are real and frightening, potentially deadly, and can cause widespread fear of particular foods. Think of Romaine lettuce spreading E. coli last year— an outbreak that infected more than 500 people and killed eight—or peanut butter spreading salmonella in 2008, which infected 167 people.
The technologies available to detect and prevent the next foodborne disease outbreak have improved greatly over the past 30-plus years, particularly during the past decade, and better, more nimble technologies are being developed, according to experts in government, academia, and private industry. The key to advancing detection of harmful foodborne pathogens, they say, is increasing speed and portability of detection, and the precision of that detection.
Getting to Rapid Results
Researchers at Purdue University have recently developed a lateral flow assay that, with the help of a laser, can detect toxins and pathogenic E. coli. Lateral flow assays are cheap and easy to use; a good example is a home pregnancy test. You place a liquid or liquefied sample on a piece of paper designed to detect a single substance and soon after you get the results in the form of a colored line: yes or no.
"They're a great portable tool for us for food contaminant detection," says Carmen Gondhalekar, a fifth-year biomedical engineering graduate student at Purdue. "But one of the areas where paper-based lateral flow assays could use improvement is in multiplexing capability and their sensitivity."
J. Paul Robinson, a professor in Purdue's Colleges of Veterinary Medicine and Engineering, and Gondhalekar's advisor, agrees. "One of the fundamental problems that we have in detection is that it is hard to identify pathogens in complex samples," he says.
When it comes to foodborne disease outbreaks, you don't always know what substance you're looking for, so an assay made to detect only a single substance isn't always effective. The goal of the project at Purdue is to make assays that can detect multiple substances at once.
These assays would be more complex than a pregnancy test. As detailed in Gondhalekar's recent paper, a laser pulse helps create a spectral signal from the sample on the assay paper, and the spectral signal is then used to determine if any unique wavelengths associated with one of several toxins or pathogens are present in the sample. Though the handheld technology has yet to be built, the idea is that the results would be given on the spot. So someone in the field trying to track the source of a Salmonella infection could, for instance, put a suspected lettuce sample on the assay and see if it has the pathogen on it.
"What our technology is designed to do is to give you a rapid assessment of the sample," says Robinson. "The goal here is speed."
Seeing the Pathogen in "High-Def"
"One in six Americans will get a foodborne illness every year," according to Dr. Heather Carleton, a microbiologist at the Centers for Disease Control and Prevention's Enteric Diseases Laboratory Branch. But not every foodborne outbreak makes the news. In 2017 alone, the CDC monitored between 18 and 37 foodborne poison clusters per week and investigated 200 multi-state clusters. Hardboiled eggs, ground beef, chopped salad kits, raw oysters, frozen tuna, and pre-cut melon are just a taste of the foods that were investigated last year for different strains of listeria, salmonella, and E. coli.
At the heart of the CDC investigations is PulseNet, a national network of laboratories that uses DNA fingerprinting to detect outbreaks at local and regional levels. This is how it works: When a patient gets sick—with symptoms like vomiting and fever, for instance—they will go to a hospital or clinic for treatment. Since we're talking about foodborne illnesses, a clinician will likely take a stool sample from the patient and send it off to a laboratory to see if there is a foodborne pathogen, like salmonella, E. Coli, or another one. If it does contain a potentially harmful pathogen, then a bacterial isolate of that identified sample is sent to a regional public health lab so that whole genome sequencing can be performed.
Whole genome sequencing can differentiate "virtually all" strains of foodborne pathogens, no matter the species, according to the FDA.
Whole genome sequencing is a method for reading the entire genome of a bacterial isolate (or from any organism, for that matter). Instead of working with a couple dozen data points, now you're working with millions of base pairs. Carleton likes to describe it as "going from an eight-bit image—maybe like what you would see in Minecraft—to a high definition image," she says. "It's really an evolution of how we detect foodborne illnesses and identify outbreaks."
If the bacterial isolate matches another in the CDC's database, this means there could be a potential outbreak and an investigation may be started, with the goal of tracking the pathogen to its source.
Whole genome sequencing has been a relatively recent shift in foodborne disease detection. For more than 20 years, the standard technique for analyzing pathogens in foodborne disease outbreaks was pulsed-field gel electrophoresis. This method creates a DNA fingerprint for each sample in the form of a pattern of about 15-30 "bands," with each band representing a piece of DNA. Researchers like Carleton can use this fingerprint to see if two samples are from the same bacteria. The problem is that 15-30 bands are not enough to differentiate all isolates. Some isolates whose bands look very similar may actually come from different sources and some whose bands look different may be from the same source. But if you can see the entire DNA fingerprint, then you don't have that issue. That's where whole genome sequencing comes in.
Although the PulseNet team had piloted whole genome sequencing as early as 2013, it wasn't until July of last year that the transition to using whole genome sequencing for all pathogens was complete. Though whole genome sequencing requires far more computing power to generate, analyze, and compare those millions of data points, the payoff is huge.
Stopping Outbreaks Sooner
The U.S. Food and Drug Administration (FDA) acquired their first whole genome sequencers in 2008, according to Dr. Eric Brown, the Director of the Division of Microbiology in the FDA's Office of Regulatory Science. Since then, through their GenomeTrakr program, a network of more than 60 domestic and international labs, the FDA has sequenced and publicly shared more than 400,000 isolates. "The impact of what whole genome sequencing could do to resolve a foodborne outbreak event was no less impactful than when NASA turned on the Hubble Telescope for the first time," says Brown.
Whole genome sequencing has helped identify strains of Salmonella that prior methods were unable to differentiate. In fact, whole genome sequencing can differentiate "virtually all" strains of foodborne pathogens, no matter the species, according to the FDA. This means it takes fewer clinical cases—fewer sick people—to detect and end an outbreak.
And perhaps the largest benefit of whole genome sequencing is that these detailed sequences—the millions of base pairs—can imply geographic location. The genomic information of bacterial strains can be different depending on the area of the country, helping these public health agencies eventually track the source of outbreaks—a restaurant, a farm, a food-processing center.
Coming Soon: "Lab in a Backpack"
Now that whole genome sequencing has become the go-to technology of choice for analyzing foodborne pathogens, the next step is making the process nimbler and more portable. Putting "the lab in a backpack," as Brown says.
The CDC's Carleton agrees. "Right now, the sequencer we use is a fairly big box that weighs about 60 pounds," she says. "We can't take it into the field."
A company called Oxford Nanopore Technologies is developing handheld sequencers. Their devices are meant to "enable the sequencing of anything by anyone anywhere," according to Dan Turner, the VP of Applications at Oxford Nanopore.
"The sooner that we can see linkages…the sooner the FDA gets in action to mitigate the problem and put in some kind of preventative control."
"Right now, sequencing is very much something that is done by people in white coats in laboratories that are set up for that purpose," says Turner. Oxford Nanopore would like to create a new, democratized paradigm.
The FDA is currently testing these types of portable sequencers. "We're very excited about it. We've done some pilots, to be able to do that sequencing in the field. To actually do it at a pond, at a river, at a canal. To do it on site right there," says Brown. "This, of course, is huge because it means we can have real-time sequencing capability to stay in step with an actual laboratory investigation in the field."
"The timeliness of this information is critical," says Marc Allard, a senior biomedical research officer and Brown's colleague at the FDA. "The sooner that we can see linkages…the sooner the FDA gets in action to mitigate the problem and put in some kind of preventative control."
At the moment, the world is rightly focused on COVID-19. But as the danger of one virus subsides, it's only a matter of time before another pathogen strikes. Hopefully, with new and advancing technology like whole genome sequencing, we can stop the next deadly outbreak before it really gets going.

