With U.S. infrastructure crumbling, an honor oath summons engineers to do no harm

When graduating college this month, many North American engineering students will take a special pledge, with a history dating back to 1925.
This spring, just like any other year, thousands of young North American engineers will graduate from their respective colleges ready to start erecting buildings, assembling machinery, and programming software, among other things. But before they take on these complex and important tasks, many of them will recite a special vow stating their ethical obligations to society, not unlike the physicians who take their Hippocratic Oath, affirming their ethos toward the patients they would treat. At the end of the ceremony, the engineers receive an iron ring, as a reminder of their promise to the millions of people their work will serve.
The ceremony isn’t just another graduation formality. As a profession, engineering has ethical weight. Moreover, engineering mistakes can be even more deadly than medical ones. A doctor’s error may cost a patient their life. But an engineering blunder may bring down a plane or crumble a building, resulting in many more fatalities. When larger projects—such as fracking, deep-sea mining or building nuclear reactors—malfunction and backfire, they can cause global disasters, afflicting millions. A vow that reminds an engineer that their work directly affects humankind and their planet is no less important than a medical oath that summons one to do no harm.
The tradition of taking an engineering oath began over a century ago in Canada. In 1922, Herbert E.T. Haultain, professor of mining engineering at the University of Toronto, presented the idea at the annual meeting of the Engineering Institute of Canada. The seven past presidents of that body were in attendance, heard Haultain’s speech and accepted his suggestion to form a committee to create an honor oath. Later, they formed the nonprofit Corporation of the Seven Wardens, which would oversee the ritual. Next year, in 1923, with the encouragement of the Seven Wardens, Haultain wrote to poet and writer Rudyard Kipling, asking him to develop a professional oath for engineers. “We are a tribe—a very important tribe within the community,” Haultain said in the letter, “but we are lacking in tribal spirit, or perhaps I should say, in manifestation of tribal spirit. Also, we are inarticulate. Can you help us?”
While Kipling is most famous now for “The Jungle Book” and perhaps his poem “Gunga Din,” he had also written a short story about engineers, “The Bridge Builders.” His poem “The Sons of Martha” can be read as a celebration of engineers:
It is their care in all the ages to take the buffet and cushion the shock.
It is their care that the gear engages; it is their care that the switches lock.
It is their care that the wheels run truly; it is their care to embark and entrain,
Tally, transport, and deliver duly the Sons of Mary by land and main.
Kipling accepted the ask and wrote the Ritual of the Calling of an Engineer, which he sent to Haultain a month later. In his response to Haultain, he stated that he preferred the word “Obligation” to “Oath.” He wrote the Obligation using Old English lettering and the old-fashioned capitalization. Kipling’s Obligation binds engineers upon their “Honor and Cold Iron” to not “suffer or pass, or be privy to the passing of, Bad Workmanship or Faulty Material,” and pardon is asked “in the presence of my betters and my equals in my Calling” for the engineer’s “assured failures and derelictions.” The hope is that when one is tempted to shoddy work by weakness or weariness, the memory of the Obligation “and the company before whom it was entered into, may return to me to aid, comfort, and restrain.”
Using the Obligation, The Seven Wardens created an induction ceremony, which seeks to unify the profession and recognize engineering’s ethics, including responsibility to the public and the need to make the best decisions possible. The induction ceremony included recitation of Kipling’s “Obligation” and incorporated an anvil, a hammer, an iron chain, and an iron ring. The inductee engineers sat inside an area marked off by the iron chain, with their more senior colleagues outside that area. At the start of the ritual, the leader beat out S-S-T in Morse code with the hammer and anvil—the letters standing for Steel, Stone, and Time. A more experienced and previously obligated engineer placed the ring on the small finger of the inductee engineer’s working hand. As per Kipling, the ring’s rough, faceted texture symbolized “the young engineer’s mind” and the difficulties engineers face in mastering their discipline.
A persistent myth purports that the original iron rings were made from the beams or bolts of the Quebec Bridge that failed twice during construction.
The first induction ceremony took place on April 25, 1925, in Montreal to obligate two of the Seven Wardens, along with four graduates from the University of Toronto class of 1893. On May 1 of that year, 14 more engineers were obligated at the University of Toronto. From that time to today most Canadian professional engineers have gone through that same ritual in their various camps, called Kipling camps—local chapters associated with various Canadian universities.
Henry Petroski, Duke University’s professor of civil engineering and history, notes in his book, “Forgive Design: Understanding Failure,” that Kipling’s poem “Sons of Martha” is often read as part of the ritual. However, sometimes inductees read Kipling’s “Hymn of Breaking Strain,” instead, which graphically depicts disastrous outcomes of engineering mistakes. The first stanza of that poem says:
The careful text-books measure
(Let all who build beware!)
The load, the shock, the pressure
Material can bear.
So, when the buckled girder
Lets down the grinding span,
'The blame of loss, or murder,
Is laid upon the man.
Not on the Stuff—the Man!
As if to strengthen the importance of these concepts, a persistent myth purports that the original iron rings were made from the beams or bolts of the Quebec Bridge that failed twice during construction. The bridge spans the St. Lawrence River upriver from Quebec City, and at the time of its construction was the world’s longest at 1,800 feet. Due to engineering errors and poor oversight, the bridge’s own weight exceeded its carrying capacity. Moreover, engineers downplayed danger when bridge beams began to warp under stress, saying that they were probably warped before they were installed. On August 29, 1907, the bridge collapsed, killing 75 of 86 workers. A second collapse occurred in 1916 when lifting equipment failed, and thirteen more workers died.
The ring myth, however, couldn’t be true. The original iron rings couldn’t have come from the failed bridge since it was made of steel, not wrought iron. Today the rings are made from stainless steel because iron deteriorates and stains engineers’ finger black.

On August 14, 2018, Morandi Bridge over Polcevera River in Genoa, Italy, collapsed from structural failure, killing 43 people.
Adobe Stock
The Seven Wardens decided to restrict the ritual to engineers trained in Canada. They copyrighted the obligation oath in Canada and the United States in 1935. Although the ritual is not a requirement for professional licensing, just like the Hippocratic Oath is not part of medical licensing, it remains a long-standing tradition.
The American Obligation of the Engineer has its own creation story, albeit a very different one. The American Order of the Engineer (OOE) was initiated in 1970, during the era of the anti-war protests, Apollo missions and the first Earth Day. On May 4, 1970, the National Guard shot into a crowd of protesters at Kent State University, killing four people. The two authors of the American obligation—Cleveland State University’s (CSU) engineering professor John Janssen and his wife Susan—reflected these historical events in the oath they wrote. Their version of the oath binds engineers to “practice integrity and fair dealing.” It also notes that their “skill carries with it the obligation to serve humanity by making the best use of the Earth’s precious wealth.” As Petroski explains in his book, “campus antiwar protestors around the country tended to view engineers as complicit in weapons proliferation [which] prompted some [CSU] engineering student leaders to look for a means of asserting some more positive values.”
Kip A. Wedel, associate professor of history and politics at Bethel College, wrote in his book, “The Obligation: A History of the Order of the Engineer,” that the ceremony was not a direct response to the Kent State shootings—it was already scheduled when the shootings happened. Yet, engineering students found the ceremony a positive action they could take in contrast to the overall turmoil. The first American ritual took place on June 4, 1970, at CSU. In total, 170 students, faculty members, and practicing engineers took the obligation. This established CSU as the first Link of the Order, as the OOE designates its local chapters. For their first ceremony, the CSU students fabricated smooth, unfaceted rings from stainless steel pipe. Later they were replaced by factory-made rings. According to Paula Ostaff, OOE’s Executive Director, about 20,000 eligible students and alumni obligate themselves yearly.
Societies hope that every engineer is imbued with a strong ethical sense and that their pledges are never far from mind. For some, the rings they wear serve a daily reminder that every paper they sign off on is touched by a physical reminder of their commitment.
These ethical and responsible engineering practices are especially salient today, when one in three American bridges needs repair or replacement, some have already collapsed, and engineers are working on projects related to the bipartisan infrastructure bill President Biden signed into law in 2021. Canada has committed $33 billion to its Investing in Canada Infrastructure Program. At the heart of these grand projects are many thousands of professional engineers, collectively working millions of hours. The professional vows they took aim to assure that the homes, bridges and airplanes they build will work as expected.
Indigenous wisdom plus honeypot ants could provide new antibiotics
Indigenous people in Australia dig pits next to a honeypot colony. Scientists think the honey can be used to make new antimicrobial drugs.
For generations, the Indigenous Tjupan people of Australia enjoyed the sweet treat of honey made by honeypot ants. As a favorite pastime, entire families would go searching for the underground colonies, first spotting a worker ant and then tracing it to its home. The ants, which belong to the species called Camponotus inflatus, usually build their subterranean homes near the mulga trees, Acacia aneura. Having traced an ant to its tree, it would be the women who carefully dug a pit next to a colony, cautious not to destroy the entire structure. Once the ant chambers were exposed, the women would harvest a small amount to avoid devastating the colony’s stocks—and the family would share the treat.
The Tjupan people also knew that the honey had antimicrobial properties. “You could use it for a sore throat,” says Danny Ulrich, a member of the Tjupan nation. “You could also use it topically, on cuts and things like that.”
These hunts have become rarer, as many of the Tjupan people have moved away and, up until now, the exact antimicrobial properties of the ant honey remained unknown. But recently, scientists Andrew Dong and Kenya Fernandes from the University of Sydney, joined Ulrich, who runs the Honeypot Ants tours in Kalgoorlie, a city in Western Australia, on a honey-gathering expedition. Afterwards, they ran a series of experiments analyzing the honey’s antimicrobial activity—and confirmed that the Indigenous wisdom was true. The honey was effective against Staphylococcus aureus, a common pathogen responsible for sore throats, skin infections like boils and sores, and also sepsis, which can result in death. Moreover, the honey also worked against two species of fungi, Cryptococcus and Aspergillus, which can be pathogenic to humans, especially those with suppressed immune systems.
In the era of growing antibiotic resistance and the rising threat of pathogenic fungi, these findings may help scientists identify and make new antimicrobial compounds. “Natural products have been honed over thousands and millions of years by nature and evolution,” says Fernandes. “And some of them have complex and intricate properties that make them really important as potential new antibiotics. “

In an era of growing resistance to antibiotics and new threats of fungi infections, the latest findings about honeypot ants are helping scientists identify new antimicrobial drugs.
Danny Ulrich
Bee honey is also known for its antimicrobial properties, but bees produce it very differently than the ants. Bees collect nectar from flowers, which they regurgitate at the hive and pack into the hexagonal honeycombs they build for storage. As they do so, they also add into the mix an enzyme called glucose oxidase produced by their glands. The enzyme converts atmospheric oxygen into hydrogen peroxide, a reactive molecule that destroys bacteria and acts as a natural preservative. After the bees pack the honey into the honeycombs, they fan it with their wings to evaporate the water. Once a honeycomb is full, the bees put a beeswax cover on it, where it stays well-preserved thanks to the enzymatic action, until the bees need it.
Less is known about the chemistry of ants’ honey-making. Similarly to bees, they collect nectar. They also collect the sweet sap of the mulga tree. Additionally, they also “milk” the aphids—small sap-sucking insects that live on the tree. When ants tickle the aphids with their antennae, the latter release a sweet substance, which the former also transfer to their colonies. That’s where the honey management difference becomes really pronounced. The ants don’t build any kind of structures to store their honey. Instead, they store it in themselves.
The workers feed their harvest to their fellow ants called repletes, stuffing them up to the point that their swollen bellies outgrow the ants themselves, looking like amber-colored honeypots—hence the name. Because of their size, repletes don’t move, but hang down from the chamber’s ceiling, acting as living feedstocks. When food becomes scarce, they regurgitate their reserves to their colony’s brethren. It’s not clear whether the repletes die afterwards or can be restuffed again. “That's a good question,” Dong says. “After they've been stretched, they can't really return to exactly the same shape.”
These replete ants are the “treat” the Tjupan women dug for. Once they saw the round-belly ants inside the chambers, they would reach in carefully and get a few scoops of them. “You see a lot of honeypot ants just hanging on the roof of the little openings,” says Ulrich’s mother, Edie Ulrich. The women would share the ants with family members who would eat them one by one. “They're very delicate,” shares Edie Ulrich—you have to take them out carefully, so they don’t accidentally pop and become a wasted resource. “Because you’d lose all this precious honey.”
Dong stumbled upon the honeypot ants phenomenon because he was interested in Indigenous foods and went on Ulrich’s tour. He quickly became fascinated with the insects and their role in the Indigenous culture. “The honeypot ants are culturally revered by the Indigenous people,” he says. Eventually he decided to test out the honey’s medicinal qualities.
The researchers were surprised to see that even the smallest, eight percent concentration of honey was able to arrest the growth of S. aureus.
To do this, the two scientists first diluted the ant honey with water. “We used something called doubling dilutions, which means that we made 32 percent dilutions, and then we halve that to 16 percent and then we half that to eight percent,” explains Fernandes. The goal was to obtain as much results as possible with the meager honey they had. “We had very, very little of the honeypot ant honey so we wanted to maximize the spectrum of results we can get without wasting too much of the sample.”
After that, the researchers grew different microbes inside a nutrient rich broth. They added the broth to the different honey dilutions and incubated the mixes for a day or two at the temperature favorable to the germs’ growth. If the resulting solution turned turbid, it was a sign that the bugs proliferated. If it stayed clear, it meant that the honey destroyed them. The researchers were surprised to see that even the smallest, eight percent concentration of honey was able to arrest the growth of S. aureus. “It was really quite amazing,” Fernandes says. “Eight milliliters of honey in 92 milliliters of water is a really tiny amount of honey compared to the amount of water.”
Similar to bee honey, the ants’ honey exhibited some peroxide antimicrobial activity, researchers found, but given how little peroxide was in the solution, they think the honey also kills germs by a different mechanism. “When we measured, we found that [the solution] did have some hydrogen peroxide, but it didn't have as much of it as we would expect based on how active it was,” Fernandes says. “Whether this hydrogen peroxide also comes from glucose oxidase or whether it's produced by another source, we don't really know,” she adds. The research team does have some hypotheses about the identity of this other germ-killing agent. “We think it is most likely some kind of antimicrobial peptide that is actually coming from the ant itself.”
The honey also has a very strong activity against the two types of fungi, Cryptococcus and Aspergillus. Both fungi are associated with trees and decaying leaves, as well as in the soils where ants live, so the insects likely have evolved some natural defense compounds, which end up inside the honey.
It wouldn’t be the first time when modern medicines take their origin from the natural world or from the indigenous people’s knowledge. The bark of the cinchona tree native to South America contains quinine, a substance that treats malaria. The Indigenous people of the Andes used the bark to quell fever and chills for generations, and when Europeans began to fall ill with malaria in the Amazon rainforest, they learned to use that medicine from the Andean people.
The wonder drug aspirin similarly takes its origin from a bark of a tree—in this case a willow.
Even some anticancer compounds originated from nature. A chemotherapy drug called Paclitaxel, was originally extracted from the Pacific yew trees, Taxus brevifolia. The samples of the Pacific yew bark were first collected in 1962 by researchers from the United States Department of Agriculture who were looking for natural compounds that might have anti-tumor activity. In December 1992, the FDA approved Paclitaxel (brand name Taxol) for the treatment of ovarian cancer and two years later for breast cancer.
In the era when the world is struggling to find new medicines fast enough to subvert a fungal or bacterial pandemic, these discoveries can pave the way to new therapeutics. “I think it's really important to listen to indigenous cultures and to take their knowledge because they have been using these sources for a really, really long time,” Fernandes says. Now we know it works, so science can elucidate the molecular mechanisms behind it, she adds. “And maybe it can even provide a lead for us to develop some kind of new treatments in the future.”
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Blood Test Can Detect Lymphoma Cells Before a Tumor Grows Back
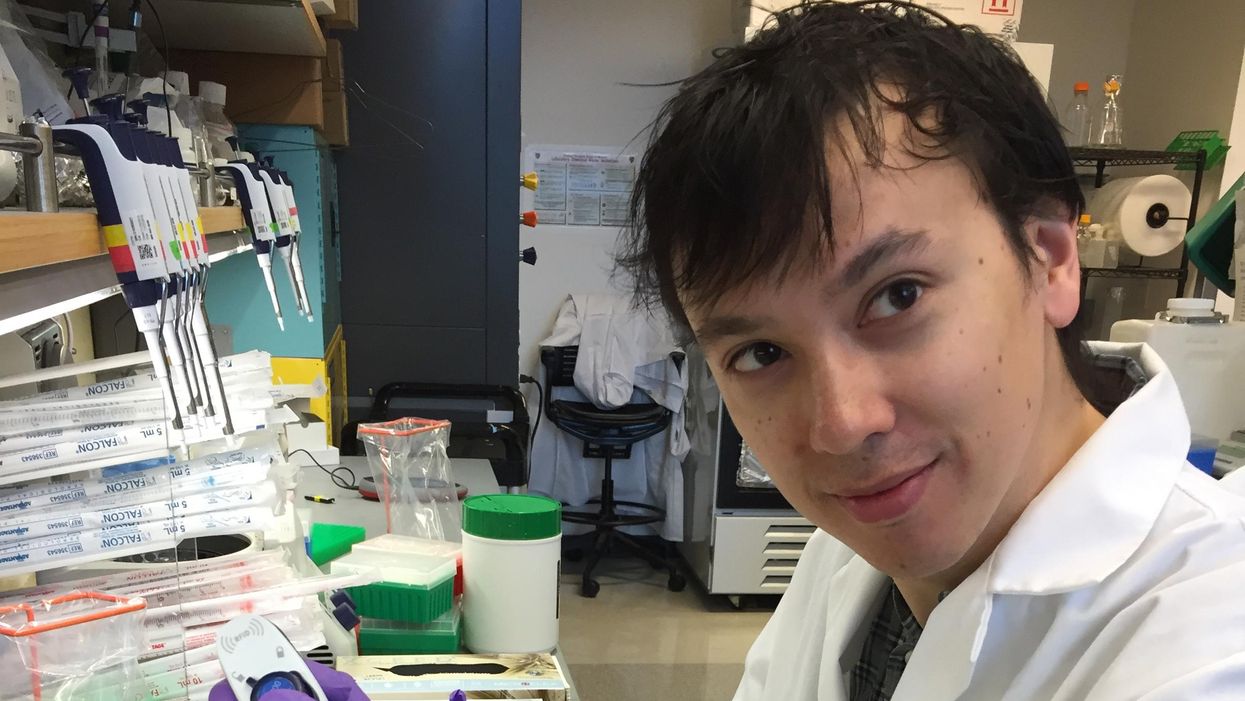
David Kurtz making DNA sequencing libraries in his lab.
When David M. Kurtz was doing his clinical fellowship at Stanford University Medical Center in 2009, specializing in lymphoma treatments, he found himself grappling with a question no one could answer. A typical regimen for these blood cancers prescribed six cycles of chemotherapy, but no one knew why. "The number seemed to be drawn out of a hat," Kurtz says. Some patients felt much better after just two doses, but had to endure the toxic effects of the entire course. For some elderly patients, the side effects of chemo are so harsh, they alone can kill. Others appeared to be cancer-free on the CT scans after the requisite six but then succumbed to it months later.
"Anecdotally, one patient decided to stop therapy after one dose because he felt it was so toxic that he opted for hospice instead," says Kurtz, now an oncologist at the center. "Five years down the road, he was alive and well. For him, just one dose was enough." Others would return for their one-year check up and find that their tumors grew back. Kurtz felt that while CT scans and MRIs were powerful tools, they weren't perfect ones. They couldn't tell him if there were any cancer cells left, stealthily waiting to germinate again. The scans only showed the tumor once it was back.
Blood cancers claim about 68,000 people a year, with a new diagnosis made about every three minutes, according to the Leukemia Research Foundation. For patients with B-cell lymphoma, which Kurtz focuses on, the survival chances are better than for some others. About 60 percent are cured, but the remaining 40 percent will relapse—possibly because they will have a negative CT scan, but still harbor malignant cells. "You can't see this on imaging," says Michael Green, who also treats blood cancers at University of Texas MD Anderson Medical Center.
The new blood test is sensitive enough to spot one cancerous perpetrator amongst one million other DNA molecules.
Kurtz wanted a better diagnostic tool, so he started working on a blood test that could capture the circulating tumor DNA or ctDNA. For that, he needed to identify the specific mutations typical for B-cell lymphomas. Working together with another fellow PhD student Jake Chabon, Kurtz finally zeroed-in on the tumor's genetic "appearance" in 2017—a pair of specific mutations sitting in close proximity to each other—a rare and telling sign. The human genome contains about 3 billion base pairs of nucleotides—molecules that compose genes—and in case of the B-cell lymphoma cells these two mutations were only a few base pairs apart. "That was the moment when the light bulb went on," Kurtz says.
The duo formed a company named Foresight Diagnostics, focusing on taking the blood test to the clinic. But knowing the tumor's mutational signature was only half the process. The other was fishing the tumor's DNA out of patients' bloodstream that contains millions of other DNA molecules, explains Chabon, now Foresight's CEO. It would be like looking for an escaped criminal in a large crowd. Kurtz and Chabon solved the problem by taking the tumor's "mug shot" first. Doctors would take the biopsy pre-treatment and sequence the tumor, as if taking the criminal's photo. After treatments, they would match the "mug shot" to all DNA molecules derived from the patient's blood sample to see if any molecular criminals managed to escape the chemo.
Foresight isn't the only company working on blood-based tumor detection tests, which are dubbed liquid biopsies—other companies such as Natera or ArcherDx developed their own. But in a recent study, the Foresight team showed that their method is significantly more sensitive in "fishing out" the cancer molecules than existing tests. Chabon says that this test can detect circulating tumor DNA in concentrations that are nearly 100 times lower than other methods. Put another way, it's sensitive enough to spot one cancerous perpetrator amongst one million other DNA molecules.
They also aim to extend their test to detect other malignancies such as lung, breast or colorectal cancers.
"It increases the sensitivity of detection and really catches most patients who are going to progress," says Green, the University of Texas oncologist who wasn't involved in the study, but is familiar with the method. It would also allow monitoring patients during treatment and making better-informed decisions about which therapy regimens would be most effective. "It's a minimally invasive test," Green says, and "it gives you a very high confidence about what's going on."
Having shown that the test works well, Kurtz and Chabon are planning a new trial in which oncologists would rely on their method to decide when to stop or continue chemo. They also aim to extend their test to detect other malignancies such as lung, breast or colorectal cancers. The latest genome sequencing technologies have sequenced and catalogued over 2,500 different tumor specimens and the Foresight team is analyzing this data, says Chabon, which gives the team the opportunity to create more molecular "mug shots."
The team hopes that that their blood cancer test will become available to patients within about five years, making doctors' job easier, and not only at the biological level. "When I tell patients, "good news, your cancer is in remission', they ask me, 'does it mean I'm cured?'" Kurtz says. "Right now I can't answer this question because I don't know—but I would like to." His company's test, he hopes, will enable him to reply with certainty. He'd very much like to have the power of that foresight.
This article is republished from our archives to coincide with Blood Cancer Awareness Month, which highlights progress in cancer diagnostics and treatment.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.