After a Diagnosis, Patients Are Finding Solace—and Empowerment—in a Sensitive Corner of Social Media

Katherine Leon and her dog enjoy nice weather in their backyard in Virginia. Leon went from feeling like she was "wandering in the woods" with doctors who hadn't experienced her spontaneous coronary artery dissection, or SCAD, to starting the world's largest registry for research on the condition.
When Kimberly Richardson of Chicago underwent chemotherapy in 2013 for ovarian cancer, her hip began to hurt. Her doctor assigned six months of physical therapy, but the pain persisted.
She took the mystery to Facebook, where she got 200 comments from cancer survivors all pointing to the same solution: Claritin. Two days after starting the antihistamine, her hip felt fine. Claritin, it turns out, reduces bone marrow swelling, a side effect of a stimulant given after chemo.
Richardson isn't alone in using social media for health. Thirty-six percent of adults with chronic diseases have benefited from health advice on the internet, or know others who have. The trend has likely accelerated during COVID-19. "With increases in anxiety and loneliness, patients find comfort in peer support," said Chris Renfro-Wallace, the chief operating officer of PatientsLikeMe, a popular online community.
Sites like PatientsLikeMe and several others are giving rise to a patient-centered view of healthcare, challenging the idea that MD stands for medical deity. They're engaging people in new ways, such as virtual clinical trials. But with misinformation spreading online about health issues, including COVID-19, there's also reason for caution.
Engaged by Design
Following her diagnosis at age 50, Richardson searched the Web. "All I saw were infographics saying in five years I'd be dead."
Eventually, she found her Facebook groups and a site called Inspire, where she met others with her rare granulosa cell tumor. "You get 15 minutes with your doctor, but on social media you can keep posting until you satisfy your question."
Virtual communities may be especially helpful for people with rarely diagnosed diseases, who wouldn't otherwise meet. When Katherine Leon of Virginia suffered chest pain after the birth of her second son, doctors said it was spontaneous coronary artery dissection, or SCAD, involving a torn artery. But she had no risk factors for heart disease. Feeling like she was "wandering in the woods" with doctors who hadn't experienced her situation, she searched online and stumbled on communities like Inspire with members who had. The experience led her to start her own Alliance and the world's largest registry for advancing research on SCAD.
"Inspire is really an extension of yourself," she said. If designed well, online sites can foster what psychologist Keith Sawyer called group mind, a dynamic where participants balance their own voices with listening to others, maximizing community engagement in health. To achieve it, participants must have what Sawyer called a "blending of egos," which may be fostered when sites let users post anonymously. They must also share goals and open communication. The latter priority has driven Brian Loew, Inspire's CEO, to safeguard the privacy of health information exchanged on the site, often asking himself, "Would I be okay if a family member had this experience?"
The vibe isn't so familial on some of Facebook's health-focused groups. There, people might sense marketers and insurers peering over their shoulders. In 2018, a researcher discovered that companies could exploit personal information on a private Facebook community for BRCA-positive women. Members of the group started a nonprofit, the Light Collective, to help peer-to-peer support platforms improve their transparency.
PatientsLikeMe and Inspire nurture the shared experience by hosting pages on scores of diseases, allowing people to better understand treatment options for multiple conditions—and find others facing the same set of issues. Four in ten American adults have more than one chronic disease.
Sawyer observed that groups are further engaged when there's a baseline of common knowledge. To that end, some platforms take care in structuring dialogues among members to promote high-quality information, stepping in to moderate when necessary. On Inspire, members get emails when others reply to their posts, instead of instant messaging. The communication lag allows staff to notice misinformation and correct it. Facebook conversations occur in real-time among many more people; "moderation is almost impossible," said Leon.
Even on PatientsLikeMe and Inspire, deciding which content to police can be tough, as variations across individuals may result in conflicting but equally valid posts. Leon's left main artery was 90 percent blocked, requiring open heart surgery, whereas others with SCAD have angina, warranting a different approach. "It's a real range of experience," she explained. "That's probably the biggest challenge: supporting everyone where they are."
Critically, these sites don't treat illnesses. "If a member asks a medical question, we typically tell them to go to their doctor," said Loew, the Inspire CEO.
Increasingly, it may be the other way around.
The Patient Will See You Now
"Some doctors embrace the idea of an educated patient," said Loew. "The more information, the better." Others, he said, aren't thrilled about patients learning on their own.
"Doctors were behind the eight ball," said Shikha Jain, an oncologist in Chicago. "We were encouraged for years to avoid social media due to patient privacy issues. There's been a drastic shift in the last few years."
Jain recently co-founded IMPACT, a grassroots organization that networks with healthcare workers across Illinois for greater awareness of health issues. She thinks doctors must meet patients where they are—increasingly, online—and learn about the various platforms where patients connect. Doctors can then suggest credible online sources for their patients' conditions. Learning about different sites takes time, Jain said, "but that's the nature of being a physician in this day and age."
At stake is the efficiency of doctor-patient interactions. "I like when patients bring in research," Jain said. "It opens up the dialogue and lets them inform the decision-making process." Richardson, the cancer survivor, agreed. "We shouldn't make the physician the villain in this conversation." Interviewed over Zoom, she was engaging but quick to challenge the assumptions behind some questions; her toughness was palpable, molded by years of fighting disease—and the healthcare system. Many doctors are forced by that system into faster office visits, she said. "If patients help their doctor get to the heart of the issue in a shorter time, now we're going down a narrower road of tests."
These conversations could be enhanced by PatientsLikeMe's Doctor Visit Guide. It uses algorithms to consolidate health data that members track on the site into a short report they can share with their physicians. "It gives the doctor a richer data set to really see how a person has been doing," said Renfro-Wallace.
Doctors aren't the only ones benefiting from these sites.
Who Profits?
A few platforms like Inspire make money by connecting their members to drug companies, so they can participate in the companies' clinical trials to test out new therapies. A cynic might say the sites are just fronts for promoting the pharmaceuticals.
The need is real, though, as many clinical trials suffer from low participation, and the experimental treatments can improve health. The key for Loew, Inspire's CEO, is being transparent about his revenue model. "When you sign up, we assume you didn't read the fine print [in the terms of agreement]." So, when Inspire tells members about openings in trials, it's a reminder the site works with pharma.
"When I was first on Inspire, all of that was invisible to me," said Leon. "It didn't dawn on me for years." Richardson believes many don't notice pharma's involvement because they're preoccupied by their medical issues.
One way Inspire builds trust is by partnering with patient advocacy groups, which tend to be nonprofit and science-oriented, said Craig Lipset, the former head of clinical innovation for Pfizer. When he developed a rare lung disease, he joined the board of a foundation that partners with Inspire's platform. The section dedicated to his disease is emblazoned with his foundation's logo and colors. Contrast that with other sites that build communities at the direct behest of drug companies, he said.
Insurance companies are also eyeing these communities. Last month, PatientsLikeMe raised $26 million in financing from investors including Optum Ventures, which belongs to the same health care company that owns a leading health insurance company, UnitedHealthcare. PatientsLikeMe is an independent company, though, and data is shared with UnitedHealth only if patients provide consent. The site is using the influx of resources to gamify improvements in health, resembling programs run by UnitedHealth that assign nutrition and fitness "missions," with apps for tracking progress. Soon, PatientsLikeMe will roll out a smarter data tracking system that gives members actionable insights and prompts them to take actions based on their conditions, as well as competitions to motivate healthier behaviors.
Such as a race to vaccinate, perhaps.
Dealing with Misinformation
An advantage of health-focused communities is the intimacy of their gatherings, compared to behemoths like Facebook. Loew, Inspire's head, is mindful of Dunbar's rule: humans can manage only about 150 friends. Inspire's social network mapping suggests many connections among members, but of different strength; Loew hopes to keep his site's familial ambiance even while expanding membership. Renfro-Wallace is exploring video and voice-only meetings to enrich the shared experiences on PatientsLikeMe, while respecting members' privacy.
But a main driver of growth and engagement online is appealing to emotion rather than reason; witness Facebook during the pandemic. "We know that misinformation and scary things spread far more rapidly than something positive," said Ann Lewandowski, the executive director of Wisconsin Immunization Neighborhood, a coalition of health providers and associations countering vaccine hesitancy across the state.
"Facebook's moderation mechanism is terrible," she said. Vaccine advocates in her region who try to flag misinformation on Facebook often have their content removed because the site's algorithm associates their posts with the distortions they're trying to warn people about.
In the realm of health, where accessing facts can mean life or death—and where ad-based revenue models conflict with privacy needs—there's probably a ceiling on how large social media sites should scale. Loew views Inspire as co-existing, not competing with Facebook.
Propagandists had months to perfect campaigns to dissuade people from mRNA vaccines. But even Lewandowski's doctor was misinformed about vaccine side effects for her condition, multiple sclerosis. She sees potential for health-focused sites to convene more virtual forums, in which patient advocacy groups educate doctors and patients on vaccine safety.
Inspire is raising awareness about COVID vaccines through a member survey with an interactive data visualization. Sampling thousands of members, the survey found vaccines are tolerated well among patients with cancer, autoimmune issues, and other serious conditions. Analytics for online groups are evolving quickly, said Lipset. "Think about the acceleration in research when you take the emerging capability for aggregating health data and mash it up with patients engaged in sharing."
Lipset recently co-founded the Decentralized Trials and Research Alliance to accelerate clinical trials and make them more accessible to patients—even from home, without risking the virus. Sites like PatientsLikeMe share this commitment, collaborating with Duke's ALS Clinic to let patients join a trial from home with just two clinic visits. Synthetic control groups were created by PatientsLikeMe's algorithms, eliminating the need for a placebo arm, enabling faster results.
As for Richardson, the ovarian cancer patient, being online has given her another type of access—to experts. She was diagnosed this year with breast cancer. "This time is totally different," she said. On Twitter, she's been direct messaging cancer researchers, whose replies have informed her disease-management strategy. When her oncologists prescribed 33 radiation treatments, she counter-proposed upping the dosage over fewer treatments. Her doctors agreed, cutting unnecessary trips from home. "I'm immuno-compromised," she said. "It's like Russian roulette. You're crossing your finger you won't get the virus."
After years of sticking up for her own health, Richardson is now positioned to look out for others. She collaborated with the University of Illinois Cancer Center on a training module that lets patients take control of their health. She's sharing it online, in a virtual community near you. "It helps you make intelligent decisions," she said. "When you speak your physician's language, it shifts the power in the room."
New tech for prison reform spreads to 11 states
The U.S. has the highest incarceration rate in the world, costing $182 billion per year, partly because its antiquated data systems often fail to identify people who should be released. A tech nonprofit is trying to change that.
A new non-profit called Recidiviz is using data technology to reduce the size of the U.S. criminal justice system. The bi-coastal company (SF and NYC) is currently working with 11 states to improve their systems and, so far, has helped remove nearly 69,000 people — ones left floundering in jail or on parole when they should have been released.
“The root cause is fragmentation,” says Clementine Jacoby, 31, a software engineer who worked at Google before co-founding Recidiviz in 2019. In the 1970s and 80s, the U.S. built a series of disconnected data systems, and this patchwork is still being used by criminal justice authorities today. It requires parole officers to manually calculate release dates, leading to errors in many cases. “[They] have done everything they need to do to earn their release, but they're still stuck in the system,” Jacoby says.
Recidiviz has built a platform that connects the different databases, with the goal of identifying people who are already qualified for release but remain behind bars or on supervision. “Think of Recidiviz like Google Maps,” says Jacoby, who worked on Maps when she was at the tech giant. Google Maps takes in data from different sources – satellite images, street maps, local business data — and organizes it into one easy view. “Recidiviz does something similar with criminal justice data,” Jacoby explains, “making it easy to identify people eligible to come home or to move to less intensive levels of supervision.”
People like Jacoby’s uncle. His experience with incarceration is what inspired her passion for criminal justice reform in the first place.
The problems are vast
The U.S. has the highest incarceration rate in the world — 2 million people according to the watchdog group, Prison Policy Initiative — at a cost of $182 billion a year. The numbers could be a lot lower if not for an array of problems including inaccurate sentencing calculations, flawed algorithms and parole violations laws.
Sentencing miscalculations
To determine eligibility for release, the current system requires corrections officers to check 21 different requirements spread across five different databases for each of the 90 to 100 people under their supervision. These manual calculations are time prohibitive, says Jacoby, and fall victim to human error.
In addition, Recidiviz found that policies aimed at helping to reduce the prison population don’t always work correctly. A key example is time off for good behavior laws that allow inmates to earn one day off for every 30 days of good behavior. Some states' data systems are built to calculate time off as one day per month of good behavior, rather than per day. Over the course of a decade-long sentence, Jacoby says these miscalculations can lead to a huge discrepancy in the calculated release data and the actual release date.
Algorithms
Commercial algorithm-based software systems for risk assessment continue to be widely used in the criminal justice system, even though a 2018 study published in Science Advances exposed their limitations. After the study went viral, it took three years for the Justice Department to issue a report on their own flawed algorithms used to reduce the federal prison population as part of the 2018 First Step Act. The program, it was determined, overestimated the risk of putting inmates of color into early-release programs.
Despite its name, Recidiviz does not build these types of algorithms for predicting recidivism, or whether someone will commit another crime after being released from prison. Rather, Jacoby says the company’s "descriptive analytics” approach is specifically intended to weed out incarceration inequalities and avoid algorithmic pitfalls.
Parole violation laws
Research shows that 350,000 people a year — about a quarter of the total prison population — are sent back not because they’ve committed another crime, but because they’ve broken a specific rule of their probation. “Things that wouldn't send you or I to prison, but would send someone on parole,” such as crossing county lines or being in the presence of alcohol when they shouldn’t be, are inflating the prison population, says Jacoby.
It’s personal for the co-founder and CEO
“I grew up with an uncle who went into the prison system,” Jacoby says. At 19, he was sentenced to ten years in prison for a non-violent crime. A few months after being released from jail, he was sent back for a non-violent parole violation.
“For my family, the fact that one in four prison admissions are driven not by a crime but by someone who's broken a rule on probation and parole was really profound because that happened to my uncle,” Jacoby says. The experience led her to begin studying criminal justice in high school, then college. She continued her dive into how the criminal justice system works as part of her Passion Project while at Google, a program that allows employees to spend 20 percent of their time on pro-bono work. Two colleagues whose family members had also been stuck in the system joined her.
As part of the project, Jacoby interviewed hundreds of people involved in the criminal justice system. “Those on the right, those on the left, agreed that bad data was slowing down reform,” she says. Their research brought them to North Dakota where they began to understand the root of the problem. The corrections department is making “huge, consequential decisions every day [without] … the data,” Jacoby says. In a new video by Recidiviz not yet released, Jacoby recounts her exchange with the state’s director of corrections who told her, “‘It’s not that we have the data and we just don’t know how to make it public; we don’t have the information you think we have.'"
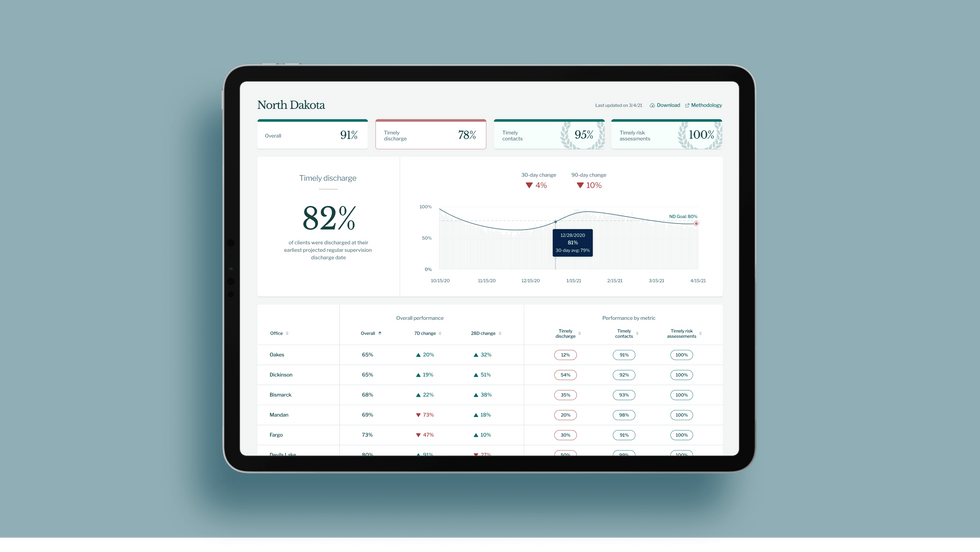
A mock-up (with fake data) of the types of dashboards and insights that Recidiviz provides to state governments.
Recidiviz
As a software engineer, Jacoby says the comment made no sense to her — until she witnessed it first-hand. “We spent a lot of time driving around in cars with corrections directors and parole officers watching them use these incredibly taxing, frankly terrible, old data systems,” Jacoby says.
As they weeded through thousands of files — some computerized, some on paper — they unearthed the consequences of bad data: Hundreds of people in prison well past their release date and thousands more whose release from parole was delayed because of minor paperwork issues. They found individuals stuck in parole because they hadn’t checked one last item off their eligibility list — like simply failing to provide their parole officer with a paystub. And, even when parolees advocated for themselves, the archaic system made it difficult for their parole officers to confirm their eligibility, so they remained in the system. Jacoby and her team also unpacked specific policies that drive racial disparities — such as fines and fees.
The Solution
It’s more than a trivial technical challenge to bring the incomplete, fragmented data onto a 21st century data platform. It takes months for Recidiviz to sift through a state’s information systems to connect databases “with the goal of tracking a person all the way through their journey and find out what’s working for 18- to 25-year-old men, what’s working for new mothers,” explains Jacoby in the video.
TED Talk: How bad data traps people in the U.S. justice system

TED Fellow Clementine Jacoby's TED Talk went live on Jan. 13. It describes how we can fix bad data in the criminal justice system, "bringing thousands of people home, reducing costs and improving public safety along the way."
Clementine Jacoby • TED2022
Ojmarrh Mitchell, an associate professor in the School of Criminology and Criminal Justice at Arizona State University, who is not involved with the company, says what Recidiviz is doing is “remarkable.” His perspective goes beyond academic analysis. In his pre-academic years, Mitchell was a probation officer, working within the framework of the “well known, but invisible” information sharing issues that plague criminal justice departments. The flexibility of Recidiviz’s approach is what makes it especially innovative, he says. “They identify the specific gaps in each jurisdiction and tailor a solution for that jurisdiction.”
On the downside, the process used by Recidiviz is “a bit opaque,” Mitchell says, with few details available on how Recidiviz designs its tools and tracks outcomes. By sharing more information about how its actions lead to progress in a given jurisdiction, Recidiviz could help reformers in other places figure out which programs have the best potential to work well.
The eleven states in which Recidiviz is working include California, Colorado, Maine, Michigan, Missouri, Pennsylvania and Tennessee. And a pilot program launched last year in Idaho, if scaled nationally, with could reduce the number of people in the criminal justice system by a quarter of a million people, Jacoby says. As part of the pilot, rather than relying on manual calculations, Recidiviz is equipping leaders and the probation officers with actionable information with a few clicks of an app that Recidiviz built.
Mitchell is disappointed that there’s even the need for Recidiviz. “This is a problem that government agencies have a responsibility to address,” he says. “But they haven’t.” For one company to come along and fill such a large gap is “remarkable.”
How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”

