Can an “old school” vaccine address global inequities in Covid-19 vaccination?

Scientists at Baylor College of Medicine developed a vaccine called Corbevax that, unlike mRNA vaccines, can be mass produced using technology already in place in low- and middle-income countries. It's now being administered in India to children aged 12-14.
When the COVID-19 pandemic began invading the world in late 2019, Peter Hotez and Maria Elena Bottazzi set out to create a low-cost vaccine that would help inoculate populations in low- and middle-income countries. The scientists, with their prior experience of developing inexpensive vaccines for the world’s poor, had anticipated that the global rollout of Covid-19 jabs would be marked with several inequities. They wanted to create a patent-free vaccine to bridge this gap, but the U.S. government did not seem impressed, forcing the researchers to turn to private philanthropies for funds.
Hotez and Bottazzi, both scientists at the Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine, raised about $9 million in private funds. Meanwhile, the U.S. government’s contribution stood at $400,000.
“That was a very tough time early on in the pandemic, you know, trying to do the work and raise the money for it at the same time,” says Hotez, who was nominated in February for a Nobel Peace Prize with Bottazzi for their COVID-19 vaccine. He adds that at the beginning of the pandemic, governments emphasized speed, innovation and rapidly immunizing populations in North America and Europe with little consideration for poorer countries. “We knew this [vaccine] was going to be the answer to global vaccine inequality, but I just wish the policymakers had felt the same,” says Hotez.
Over the past two years, the world has witnessed 488 million COVID-19 infections and over 61 million deaths. Over 11 billion vaccine doses have been administered worldwide; however, the global rollout of COVID-19 vaccines is marked with alarming socio-economic inequities. For instance, 72 percent of the population in high-income countries has received at least one dose of the vaccine, whereas the number stands at 15 percent in low-income countries.
This inequity is worsening vulnerabilities across the world, says Lawrence Young, a virologist and co-lead of the Warwick Health Global Research Priority at the UK-based University of Warwick. “As long as the virus continues to spread and replicate, particularly in populations who are under-vaccinated, it will throw up new variants and these will remain a continual threat even to those countries with high rates of vaccination,” says Young, “Therefore, it is in all our interests to ensure that vaccines are distributed equitably across the world.”
“When your house is on fire, you don't call the patent attorney,” says Hotez. “We wanted to be the fire department.”
The vaccine developed by Hotez and Bottazzi recently received emergency use authorisation in India, which plans to manufacture 100 million doses every month. Dubbed ‘Corbevax’ by its Indian maker, Biological E Limited, the vaccine is now being administered in India to children aged 12-14. The patent-free arrangement means that other low- and middle-income countries could also produce and distribute the vaccine locally.
“When your house is on fire, you don't call the patent attorney, you call the fire department,” says Hotez, commenting on the intellectual property rights waiver. “We wanted to be the fire department.”
The Inequity
Vaccine equity simply means that all people, irrespective of their location, should have equal access to vaccines. However, data suggests that the global COVID-19 vaccine rollout has favoured those in richer countries. For instance, high-income countries like the UAE, Portugal, Chile, Singapore, Australia, Malta, Hong Kong and Canada have partially vaccinated over 85 percent of their populations. This percentage in poorer countries, meanwhile, is abysmally low – 2.1 percent in Yemen, 4.6 in South Sudan, 5 in Cameroon, 9.9 in Burkina Faso, 10 in Nigeria, 12 in Somalia, 12 in Congo, 13 in Afghanistan and 21 in Ethiopia.

In late 2019, scientists Peter Hotez and Maria Elena Bottazzi set out to create a low-cost vaccine that would help inoculate populations in low- and middle-income countries. In February, they were nominated for a Nobel Peace Prize.
Texas Children's Hospital
The COVID-19 vaccination coverage is particularly low in African countries, and according to Shabir Madhi, a vaccinologist at the University of the Witwatersrand, Johannesburg and co-director of African Local Initiative for Vaccinology Expertise, vaccine access and inequity remains a challenge in Africa. Madhi adds that a lack of vaccine access has affected the pandemic’s trajectory on the continent, but a majority of its people have now developed immunity through natural infection. “This has come at a high cost of loss of lives,” he says.
COVID-19 vaccines mean a significant financial burden for poorer countries, which spend an average of $41 per capita annually on health, while the average cost of every COVID-19 vaccine dose ranges between $2 and $40 in addition to a distribution cost of $3.70 per person for two doses. In December last year, the World Health Organisation (WHO) set a goal of immunizing 70 percent of the population of all countries by mid-2022. This, however, means that low-income countries would have to increase their health expenditure by an average of 56.6 percent to cover the cost, as opposed to 0.8 per cent in high-income countries.
Reflecting on the factors that have driven global inequity in COVID-19 vaccine distribution, Andrea Taylor, assistant director of programs at the Duke Global Health Innovation Center, says that wealthy nations took the risk of investing heavily in the development and scaling up of COVID-19 vaccines – at a time when there was little evidence to show that vaccines would work. This reserved a place for these nations at the front of the queue when doses started rolling off production lines. Lower-income countries, meanwhile, could not afford such investments.
“Now, however, global supply is not the issue,” says Taylor. “We are making plenty of doses to meet global need. The main problem is infrastructure to get the vaccine where it is most needed in a predictable and timely way and to ensure that countries have all the support they need to store, transport, and use the vaccine once it is received.”

Taufique Joarder, vice-chairperson of Bangladesh's Public Health Foundation, sees the need for more trials and data before Corbevax is made available to the general population.
In addition to global inequities in vaccination coverage, there are inequities within nations. Taufique Joarder, vice-chairperson of Bangladesh’s Public Health Foundation, points to the situation in his country, where vaccination coverage in rural and economically disadvantaged communities has suffered owing to weak vaccine-promotion initiatives and the difficulty many people face in registering online for jabs.
Joarder also cites the example of the COVID-19 immunization drive for children aged 12 years and above. “[Children] are given the Pfizer vaccine, which requires an ultralow temperature for storage. This is almost impossible to administer in many parts of the country, especially the rural areas. So, a large proportion of the children are being left out of vaccination,” says Joarder, adding that Corbevax, which is cheaper and requires regular temperature refrigeration “can be an excellent alternative to Pfizer for vaccinating rural children.”
Corbevax vs. mRNA Vaccines
As opposed to most other COVID-19 vaccines, which use the new Messenger RNA (mRNA) vaccine technology, Corbevax is an “old school” vaccine, says Hotez. The vaccine is made through microbial fermentation in yeast, similar to the process used to produce the recombinant hepatitis B vaccine, which has been administered to children in several countries for decades. Hence, says Hotez, the technology to produce Corbevax at large scales is already in place in countries like Vietnam, Bangladesh, India, Indonesia, Brazil, Argentina, among many others.
“So if you want to rapidly develop and produce and empower low- and middle-income countries, this is the technology to do it,” he says.
“Global access to high-quality vaccines will require serious investment in other types of COVID-19 vaccines," says Andrea Taylor.
The COVID-19 vaccines created by Pfizer-BioNTech and Moderna marked the first time that mRNA vaccine technology was approved for use. However, scientists like Young feel that there is “a need to be pragmatic and not seduced by new technologies when older, tried and tested approaches can also be effective.” Taylor, meanwhile, says that although mRNA vaccines have dominated the COVID-19 vaccine market in the U.S., “there is no clear grounding for this preference in the data we have so far.” She adds that there is also growing evidence that the immunity from these shots may not hold up as well over time as that of vaccines using different platforms.
“The mRNA vaccines are well suited to wealthy countries with sufficient ultra-cold storage and transportation infrastructure, but these vaccines are divas and do not travel well in the rest of the world,” says Taylor. “Global access to high-quality vaccines will require serious investment in other types of COVID-19 vaccines, such as the protein subunit platform used by Novavax and Corbevax. These require only standard refrigeration, can be manufactured using existing facilities all over the world, and are easy to transport.”
Joarder adds that Corbevax is cheaper due to the developers’ waived intellectual rights. It could also be used as a booster vaccine in Bangladesh, where only five per cent of the population has currently received booster doses. “If this vaccine is proved effective for heterologous boosting, [meaning] it works well and is well tolerated as a booster with other vaccines that are available in Bangladesh, this can be useful,” says Joarder.
According to Hotez, Corbevax can play several important roles - as a standalone adult or paediatric vaccine, and as a booster for other vaccines. Studies are underway to determine Corbevax’s effectiveness in these regards, he says.
Need for More Data
Biological E conducted two clinical trials involving 3000 subjects in India, and found Corbevax to be “safe and immunogenic,” with 90 percent effectiveness in preventing symptomatic infections from the original strain of COVID-19 and over 80 percent effectiveness against the Delta variant. The vaccine is currently in use in India, and according to Hotez, it’s in the pipeline at different stages in Indonesia, Bangladesh and Botswana.
However, Corbevax is yet to receive emergency use approval from the WHO. Experts such as Joarder see the need for more trials and data before it is made available to the general population. He says that while the WHO’s emergency approval is essential for global scale-up of the vaccine, we need data to determine age-stratified efficacy of the vaccine and whether it can be used for heterologous boosting with other vaccines. “According to the most recent data, the 100 percent circulating variant in Bangladesh is Omicron. We need to know how effective is Corbevax against the Omicron variant,” says Joarder.

Shabir Madhi, a vaccinologist at the University of the Witwatersrand, Johannesburg and co-director of the African Local Initiative for Vaccinology Expertise, says that a majority of people in Africa have now developed immunity through natural infection. “This has come at a high cost of loss of lives."
Shivan Parusnath
Others, meanwhile, believe that availing vaccines to poorer countries is not enough to resolve the inequity. Young, the Warwick virologist, says that the global vaccination rollout has also suffered from a degree of vaccine hesitancy, echoing similar observations by President Biden and Pfizer’s CEO. The problem can be blamed on poor communication about the benefits of vaccination. “The Corbevax vaccine [helps with the issues of] patent protection, vaccine storage and distribution, but governments need to ensure that their people are clearly informed.” Notably, however, some research has found higher vaccine willingness in lower-income countries than in the U.S.
Young also emphasized the importance of establishing local vaccination stations to improve access. For some countries, meanwhile, it may be too late. Speaking about the African continent, Madhi says that Corbevax has arrived following the peak of the crisis and won’t reverse the suffering and death that has transpired because of vaccine hoarding by high-income countries.
“The same goes for all the sudden donations from countries such as France - pretty much of little to no value when the pandemic is at its tail end,” says Madhi. “This, unfortunately, is a repeat of the swine flu pandemic in 2009, when vaccines only became available to Africa after the pandemic had very much subsided.”
Paralyzed By Polio, This British Tea Broker Changed the Course Of Medical History Forever
Robin Cavendish in his special wheelchair with his son Jonathan in the 1960s.
In December 1958, on a vacation with his wife in Kenya, a 28-year-old British tea broker named Robin Cavendish became suddenly ill. Neither he nor his wife Diana knew it at the time, but Robin's illness would change the course of medical history forever.
Robin was rushed to a nearby hospital in Kenya where the medical staff delivered the crushing news: Robin had contracted polio, and the paralysis creeping up his body was almost certainly permanent. The doctors placed Robin on a ventilator through a tracheotomy in his neck, as the paralysis from his polio infection had rendered him unable to breathe on his own – and going off the average life expectancy at the time, they gave him only three months to live. Robin and Diana (who was pregnant at the time with their first child, Jonathan) flew back to England so he could be admitted to a hospital. They mentally prepared to wait out Robin's final days.
But Robin did something unexpected when he returned to the UK – just one of many things that would astonish doctors over the next several years: He survived. Diana gave birth to Jonathan in February 1959 and continued to visit Robin regularly in the hospital with the baby. Despite doctors warning that he would soon succumb to his illness, Robin kept living.
After a year in the hospital, Diana suggested something radical: She wanted Robin to leave the hospital and live at home in South Oxfordshire for as long as he possibly could, with her as his nurse. At the time, this suggestion was unheard of. People like Robin who depended on machinery to keep them breathing had only ever lived inside hospital walls, as the prevailing belief was that the machinery needed to keep them alive was too complicated for laypeople to operate. But Diana and Robin were up for the challenges – and the risks. Because his ventilator ran on electricity, if the house were to unexpectedly lose power, Diana would either need to restore power quickly or hand-pump air into his lungs to keep him alive.
Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
In an interview as an adult, Jonathan Cavendish reflected on his parents' decision to live outside the hospital on a ventilator: "My father's mantra was quality of life," he explained. "He could have stayed in the hospital, but he didn't think that was as good of a life as he could manage. He would rather be two minutes away from death and living a full life."
After a few years of living at home, however, Robin became tired of being confined to his bed. He longed to sit outside, to visit friends, to travel – but had no way of doing so without his ventilator. So together with his friend Teddy Hall, a professor and engineer at Oxford University, the two collaborated in 1962 to create an entirely new invention: a battery-operated wheelchair prototype with a ventilator built in. With this, Robin could now venture outside the house – and soon the Cavendish family became famous for taking vacations. It was something that, by all accounts, had never been done before by someone who was ventilator-dependent. Robin and Hall also designed a van so that the wheelchair could be plugged in and powered during travel. Jonathan Cavendish later recalled a particular family vacation that nearly ended in disaster when the van broke down outside of Barcelona, Spain:
"My poor old uncle [plugged] my father's chair into the wrong socket," Cavendish later recalled, causing the electricity to short. "There was fire and smoke, and both the van and the chair ground to a halt." Johnathan, who was eight or nine at the time, his mother, and his uncle took turns hand-pumping Robin's ventilator by the roadside for the next thirty-six hours, waiting for Professor Hall to arrive in town and repair the van. Rather than being panicked, the Cavendishes managed to turn the vigil into a party. Townspeople came to greet them, bringing food and music, and a local priest even stopped by to give his blessing.
Robin had become a pioneer, showing the world that a person with severe disabilities could still have mobility, access, and a fuller quality of life than anyone had imagined. His mission, along with Hall's, then became gifting this independence to others like himself. Robin and Hall raised money – first from the Ernest Kleinwort Charitable Trust, and then from the British Department of Health – to fund more ventilator chairs, which were then manufactured by Hall's company, Littlemore Scientific Engineering, and given to fellow patients who wanted to live full lives at home. Robin and Hall used themselves as guinea pigs, testing out different models of the chairs and collaborating with scientists to create other devices for those with disabilities. One invention, called the Possum, allowed paraplegics to control things like the telephone and television set with just a nod of the head. Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
Robin went on to enjoy a long and happy life with his family at their house in South Oxfordshire, surrounded by friends who would later attest to his "down-to-earth" personality, his sense of humor, and his "irresistible" charm. When he died peacefully at his home in 1994 at age 64, he was considered the world's oldest-living person who used a ventilator outside the hospital – breaking yet another barrier for what medical science thought was possible.
Kirstie Ennis, an Afghanistan veteran who survived a helicopter crash but lost a limb, pictured in May 2021 at Two Rivers Park in Colorado.
In June 2012, Kirstie Ennis was six months into her second deployment to Afghanistan and recently promoted to sergeant. The helicopter gunner and seven others were three hours into a routine mission of combat resupplies and troop transport when their CH-53D helicopter went down hard.
Miraculously, all eight people onboard survived, but Ennis' injuries were many and severe. She had a torn rotator cuff, torn labrum, crushed cervical discs, facial fractures, deep lacerations and traumatic brain injury. Despite a severely fractured ankle, doctors managed to save her foot, for a while at least.
In November 2015, after three years of constant pain and too many surgeries to count, Ennis relented. She elected to undergo a lower leg amputation but only after she completed the 1,000-mile, 72-day Walking with the Wounded journey across the UK.
On Veteran's Day of that year, on the other side of the country, orthopedic surgeon Cato Laurencin announced a moonshot challenge he was setting out to achieve on behalf of wounded warriors like Ennis: the Hartford Engineering A Limb (HEAL) Project.
Laurencin, who is a University of Connecticut professor of chemical, materials and biomedical engineering, teamed up with experts in tissue bioengineering and regenerative medicine from Harvard, Columbia, UC Irvine and SASTRA University in India. Laurencin and his colleagues at the Connecticut Convergence Institute for Translation in Regenerative Engineering made a bold commitment to regenerate an entire limb within 15 years – by the year 2030.

Dr. Cato Laurencin pictured in his office at UConn.
Photo Credit: UConn
Regenerative Engineering -- A Whole New Field
Limb regeneration in humans has been a medical and scientific fascination for decades, with little to show for the effort. However, Laurencin believes that if we are to reach the next level of 21st century medical advances, this puzzle must be solved.
An estimated 185,000 people undergo upper or lower limb amputation every year. Despite the significant advances in electromechanical prosthetics, these individuals still lack the ability to perform complex functions such as sensation for tactile input, normal gait and movement feedback. As far as Laurencin is concerned, the only clinical answer that makes sense is to regenerate a whole functional limb.
Laurencin feels other regeneration efforts were hampered by their siloed research methods with chemists, surgeons, engineers all working separately. Success, he argues, requires a paradigm shift to a trans-disciplinary approach that brings together cutting-edge technologies from disparate fields such as biology, material sciences, physical, chemical and engineering sciences.
As the only surgeon ever inducted into the academies of Science, Medicine and Innovation, Laurencin is uniquely suited for the challenge. He is regarded as the founder of Regenerative Engineering, defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems.
But none of this is achievable without early clinician participation across scientific fields to develop new technologies and a deeper understanding of how to harness the body's innate regenerative capabilities. "When I perform a surgical procedure or something is torn or needs to be repaired, I count on the body being involved in regenerating tissue," he says. "So, understanding how the body works to regenerate itself and harnessing that ability is an important factor for the regeneration process."
The Birth of the Vision
Laurencin's passion for regeneration began when he was a sports medicine fellow at Cornell University Medical Center in the early 1990s. There he saw a significant number of injuries to the anterior cruciate ligament (ACL), the major ligament that stabilizes the knee. He believed he could develop a better way to address those injuries using biomaterials to regenerate the ligament. He sketched out a preliminary drawing on a napkin one night over dinner. He has spent the next 30 years regenerating tissues, including the patented L-C ligament.
As chair of Orthopaedic Surgery at the University of Virginia during the peak of the wars in Iraq and Afghanistan, Laurencin treated military personnel who survived because of improved helmets, body armor and battlefield medicine but were left with more devastating injuries, including traumatic brain injuries and limb loss.
"I was so honored to care for them and I so admired their steadfast courage that I became determined to do something big for them," says Laurencin.
When he tells people about his plans to regrow a limb, he gets a lot of eye rolls, which he finds amusing but not discouraging. Growing bone cells was relatively new when he was first focused on regenerating bone in 1987 at MIT; in 2007 he was well on his way to regenerating ligaments at UVA when many still doubted that ligaments could even be reconstructed. He and his team have already regenerated torn rotator cuff tendons and ACL ligaments using a nano-textured fabric seeded with stem cells.
Even as a finalist for the $4 million NIH Pioneer Award for high-risk/high-reward research, he faced a skeptical scientific audience in 2014. "They said, 'Well what do you plan to do?' I said 'I plan to regenerate a whole limb in people.' There was a lot of incredulousness. They stared at me and asked a lot of questions. About three days later, I received probably the best score I've ever gotten on an NIH grant."
In the Thick of the Science
Humans are born with regenerative abilities--two-year-olds have regrown fingertips--but lose that ability with age. Salamanders are the only vertebrates that can regenerate lost body parts as adults; axolotl, the rare Mexican salamander, can grow extra limbs.
The axolotl is important as a model organism because it is a four-footed vertebrate with a similar body plan to humans. Mapping the axolotl genome in 2018 enhanced scientists' genetic understanding of their evolution, development, and regeneration. Being easy to breed in captivity allowed the HEAL team to closely study these amphibians and discover a new cell type they believe may shed light on how to mimic the process in humans.
"Whenever limb regeneration takes place in the salamander, there is a huge amount of something called heparan sulfate around that area," explains Laurencin. "We thought, 'What if this heparan sulfate is the key ingredient to allowing regeneration to take place?' We found these groups of cells that were interspersed in tissues during the time of regeneration that seemed to have connections to each other that expressed this heparan sulfate."
Called GRID (Groups that are Regenerative, Interspersed and Dendritic), these cells were also recently discovered in mice. While GRID cells don't regenerate as well in mice as in salamanders, finding them in mammals was significant.
"If they're found in mice. we might be able to find these in humans in some form," Laurencin says. "We think maybe it will help us figure out regeneration or we can create cells that mimic what grid cells do and create an artificial grid cell."
What Comes Next?
Laurencin and his team have individually engineered and made every single tissue in the lower limb, including bone, cartilage, ligament, skin, nerve, blood vessels. Regenerating joints and joint tissue is the next big mile marker, which Laurencin sees as essential to regenerating a limb that functions and performs in the way he envisions.
"Using stem cells and amnion tissue, we can regenerate joints that are damaged, and have severe arthritis," he says. "We're making progress on all fronts, and making discoveries we believe are going to be helping people along the way."
That focus and advancement is vital to Ennis. After laboring over the decision to have her leg amputated below the knee, she contracted MRSA two weeks post-surgery. In less than a month, she went from a below-the-knee-amputee to a through-the-knee amputee to an above-the-knee amputee.
"A below-the-knee amputation is night-and-day from above-the-knee," she said. "You have to relearn everything. You're basically a toddler."
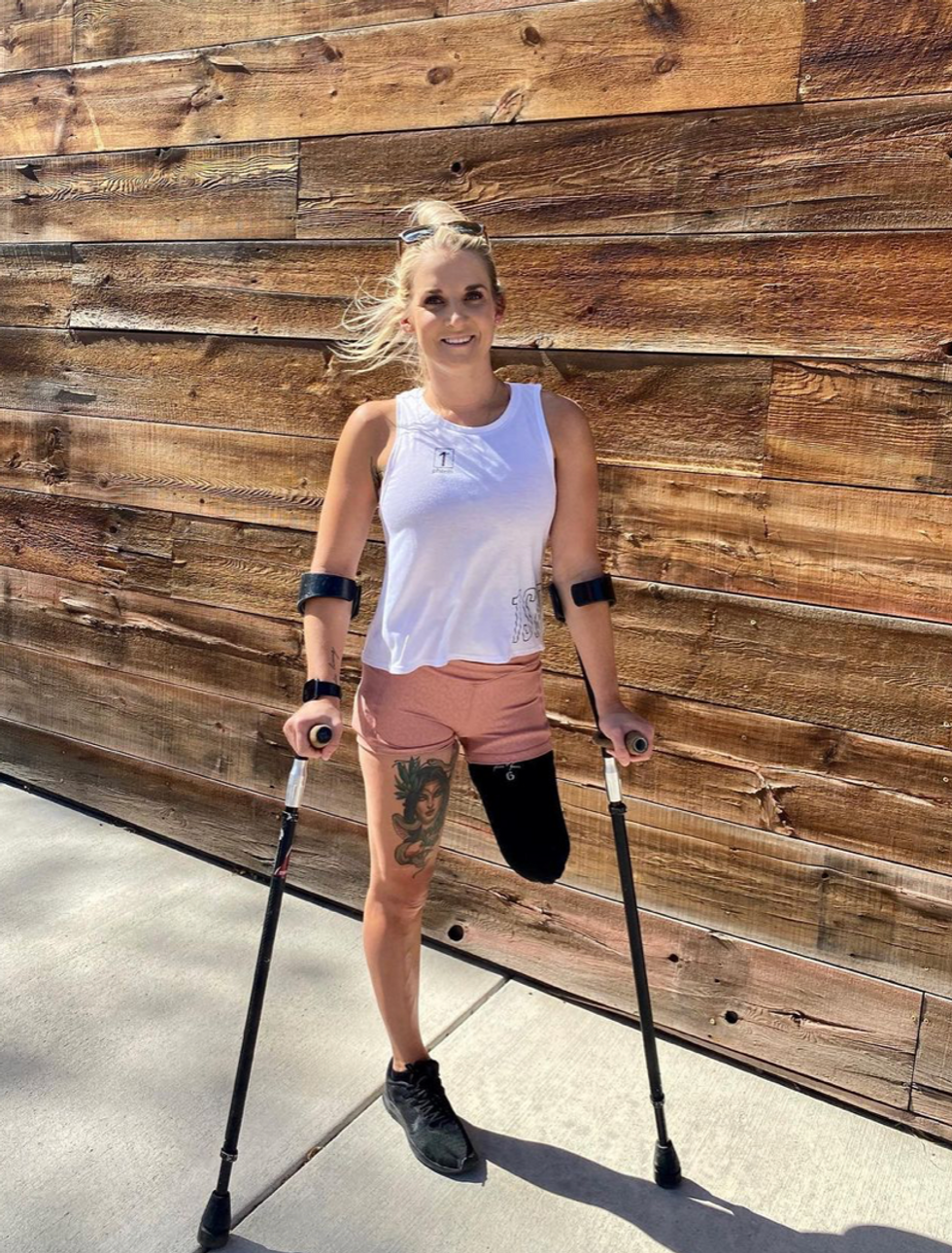
Kirstie Ennis pictured in July 2020.
Photo Credit: Ennis' Instagram
The clock is ticking on the timeline Laurencin set for himself. Nine years might seem like forever if you're doing time but it might appear fleeting when you're trying to create something that's never been done before. But Laurencin isn't worried. He's convinced time is on his side.
"Every week, I receive an email or a call from someone, maybe a mother whose child has lost a finger or I'm in communication with a disabled American veteran who wants to know how the progress is going. That energizes me to continue to work hard to try to create these sorts of solutions because we're talking about people and their lives."
He devotes about 60 hours a week to the project and the roughly 100 students, faculty and staff who make up the HEAL team at the Convergence Institute seem acutely aware of what's at stake and appear equally dedicated.
"We're in the thick of the science in terms of making this happen," says Laurencin. "We've moved from making the impossible possible to making the possible a reality. That's what science is all about."

