Six Questions about the Kids' COVID Vaccine, Answered by an Infectious Disease Doctor

The author, an infectious disease physician, pictured with his two daughters who are getting vaccinated against COVID-19.
I enthusiastically support the vaccination against COVID for children aged 5-11 years old. As an infectious disease doctor who took care of hundreds of COVID-19 patients over the past 20 months, I have seen the immediate and long-term consequences of COVID-19 on patients – and on their families. As a father of two daughters, I have lived through the fear and anxiety of protecting my kids at all cost from the scourges of the pandemic and worried constantly about bringing the virus home from work.
It is imperative that we vaccinate as many children in the community as possible. There are several reasons why. First children do get sick from COVID-19. Over the course of the pandemic in the U.S, more than 2 million children aged 5-11 have become infected, more than 8000 have been hospitalized, and more than 100 have died, making COVID one of the top 10 causes of pediatric deaths in this age group over the past year. Children are also susceptible to chronic consequences of COVID such as long COVID and multisystem inflammatory syndrome in children (MIS-C). Most studies demonstrate that 10-30% of children will develop chronic symptoms following COVID-19. These include complaints of brain fog, fatigue, trouble breathing, fever, headache, muscle and joint pains, abdominal pain, mood swings and even psychiatric disorders. Symptoms typically last from 4-8 weeks in children, with some reporting symptoms that persist for many months.
Second, children are increasingly recognized as vectors who can bring infection into the house, potentially transmitting infection to vulnerable household members. Finally, we have all seen the mayhem that results when one child in the classroom becomes infected with COVID and the other students get sent home to quarantine – across the U.S., more than 2000 schools have been affected this way.
We now have an extraordinarily effective vaccine with more than 90 percent efficacy at preventing symptomatic infection. Vaccinating children will boost our countrywide vaccination rate which is trailing many countries after an early start. Nevertheless, there are still many questions and concerns that parents have as the vaccine gets rolled out. I will address six of them here.
"Novel Vaccine Technology"
Even though this is a relatively new vaccine, the technology is not new. Scientists had worked on mRNA vaccines for decades prior to the COVID mRNA vaccine breakthrough. Furthermore, experience with the Pfizer COVID vaccine is rapidly growing. By now it has been more than a year and a half since the Pfizer trials began in March 2020, and more than 7 billion doses have already been administered globally, including in 13.7 million adolescents in the U.S. alone.
"Will This Vaccine Alter My Child's DNA?"
No. This is not how mRNA works. DNA is present in the cell's nucleus. The mRNA only stays in the outside cytoplasm, gets destroyed and never enters the inner sanctum of the nucleus. Furthermore, for the mRNA to be ever integrated into DNA, it requires a special enzyme called reverse transcriptase which humans don't have. Proteins (that look like the spike proteins on SARS-CoV-2) are made directly from this mRNA message without involvement of our DNA at any time. Pieces of spike proteins get displayed on the outside of our cells and our body makes protective antibodies that then protects us handily against the future real virus if it were ever to enter our (or our children's) bodies. Our children's DNA or genes can never be affected by an mRNA vaccine.
"Lack of Info on Long-Term Side Effects"
Unlike medications that are taken daily or periodically and can build up over time, the mRNA in the Pfizer vaccine is evanescent. It literally is just the messenger (that is what the "m" in mRNA stands for) and the messenger quickly disappears. mRNA is extremely fragile and easily inactivated – that's why we need to encase it in a special fatty bubble and store the vaccines at extremely cold temperatures. Our cells break down and destroy the mRNA within a few days after receiving the instructions to make the virus spike proteins. The presence of these fragments of the virus (note this is not "live" virus) prompts our immune system to generate protective antibodies to the real thing. Our bodies break down mRNA all the time in normal cellular processes – this is nothing new.
What the transience of the delivery system means is that most of the effects of the mRNA vaccines are expected to be more immediate (sore arm, redness at the site, fever, chills etc.), with no long-term side effects anticipated. A severe allergic response has been reported to occur in some generally within the first 15 minutes, is very rare, and everyone gets observed for that as part of standard vaccine administration. Even with the very uncommon complication of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) seen primarily in young men under the age of 30 following mRNA vaccines, these typically happen within days to 2 weeks and many return to work or school in days. In the 70-year history of pediatric (and adult vaccines), dangerous complications happen in the first two months. There have been millions of adolescents as young as 12 years and thousands in the initial trial of children aged 5-11 who have already received the vaccine and are well beyond the two-month period of observation. There is no biological reason to believe that younger children will have a different long-term side effect profile compared to adolescents or adults.
"Small Sample Size in Kids and the Trial Design"
Although the Pfizer trial in children aged 5-11 was relatively small, it was big enough to give us statistical confidence in assessing safety and efficacy outcomes. Scientists spend a lot of time determining the right sample size of a study during the design phase. On one hand, you want to conduct the study efficiently so that resources are used in a cost-effective way and that you get a timely answer, especially in a fast-moving pandemic. On the other hand, you want to make sure you have enough sample size so that you can answer the question confidently as to whether the intervention works and whether there are adverse effects. The more profound the effect size of the intervention (in this case the vaccine), the fewer the numbers of children needed in the trials.
Statistics help investigators determine whether the results seen would have appeared by chance or not. In this case, the effect was real and impressive. Over 3,000 children around the world have received the vaccines through the trials alone with no serious side effects detected. The first press release reported that the immune response in children aged 5-11 was similar (at one-third the vaccine dose) to the response in the comparator group aged 16-25 years old. Extrapolating clinical efficacy results from immune response measurements ("immunobridging" study) would already have been acceptable if this was the only data. This is a standard trial design for many pediatric vaccines. Vaccines are first tested in the lab, followed by animals then adults. Only when deemed safe in adults and various regulatory bodies have signed off, do the pediatric vaccine trials commence.
Because children's immune systems and bodies are in a constant state of development, the vaccines must be right-sized. Investigators typically conduct "age de-escalation" studies in various age groups. The lowest dose is first tried so see if that is effective, then the dose is increased gradually as needed. Immune response is the easiest, safest and most efficient way to test the efficacy of pediatric vaccines. This is a typical size and design of a childhood vaccine seeking regulatory approval. There is no reason to think that the clinical efficacy would be any different in children vs. adults for a given antibody response, given the experience already in the remainder of the population, including older children and adolescents. Although this was primarily designed as an "immunobridging" study, the initial immunologic response data was followed by real clinical outcomes in this population. Reporting on the outcomes of 2,268 children in the randomized controlled trial, the vaccine was 90.7% effective at preventing symptomatic infection.
"Fear of Myocarditis"
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) have been associated with receipt of the mRNA vaccines, particularly among male adolescents and young adults, typically within a few days after receiving the second dose. But this is very rare. For every million vaccine recipients, you would expect 41 cases in males, and 4 cases in females aged 12-29 years-old. The risk in older age groups is substantially lower. It is important to recognize that the risk of myocarditis associated with COVID is substantially higher. Patients present with new chest pain, shortness of breath, or palpitations after receiving an mRNA vaccine (more common after the second dose). But outcomes are good if associated with the vaccine. Most respond well to treatment and resolve symptoms within a week. There have been no deaths associated with vaccine-associated myocarditis.
In contrast, COVID-associated myocarditis has been associated with more severe cases as well as other complications including chronic symptoms of long COVID. The risk of myocarditis is likely related to vaccine dose, so the fact that one-third the dose of the vaccine will be used in the 5-11 year-olds is expected to correspond to a lower risk of myocarditis. At the lower dose given to younger kids, there has been a lower incidence of adverse effects reported compared to older children and adults who received the full dose. In addition, baseline rates of myocarditis not associated with vaccination are much lower in children ages 5-11 years than in older children, so the same may hold true for vaccine-associated myocarditis cases. This is because myocarditis is associated with sex hormones (particularly testosterone) that surge during puberty. In support of this, the incidence of vaccine-associated myocarditis is lower in 12–15-year-old boys, compared to those who were older than 16 years old. There were no cases of myocarditis reported in the experience to date of 5–11-year-old children in the trials, although the trial was too small to pick up on such a rare effect.
"Optimal Dose Spacing Interval: Longer Than 3 Weeks?"
There is a biologic basis for increasing the interval between vaccine doses in general. Priming the immune system with the first shot and then waiting gives the second shot a better chance of prompting a secondary immune reaction that results in a more durable response (with more T cell driven immune memory). One study from the U.K. showed that the antibody response in people over 80 was more than 3 times higher if they delayed the second dose to after 12 weeks for the Pfizer vaccine instead of the 3 weeks studied in trials. In a study of 503 British health care workers, there were twice as many neutralizing antibodies produced in a longer interval group (6-14 weeks) versus a shorter interval group (3-4 weeks) between doses. However, the safety and efficacy with longer intervals has not been evaluated in the pediatric or other COVID vaccine trials.
In the U.S., the C.D.C. reported that 88 percent of counties are at a "high" or "substantial" level of community transmission. Also, Europe is already experiencing a winter surge of infections that may predict more U.S. winter cases as international travel reopens. During a time of high community virus burden with a highly transmissible Delta variant, relying on one dose of vaccine for several more weeks until the second may leave many more susceptible to infection while waiting. One study from England showed that one dose of the Pfizer vaccine was only 33% protective against symptomatic Delta infection in contrast to 50% for the Alpha variant in adults. There has been no corollary information in children but we would expect less protection in general from one vaccine dose vs. two. This is a particularly important issue with the upcoming holiday season when an increased number of families will travel. Some countries such as the U.K. and Norway have proceeded with only offering older than 12 year-olds one dose of vaccine rather than two, but this was before the current European surge which may change the risk-benefit calculus. There are no plans to only offer one vaccine dose in the U.S. at this time. However a lower dose of the vaccine will likely be studied in the future for adolescents aged 12-15.
For parents worried about the potential risk of adverse effects of two doses of vaccines in their children, it is reasonable to wait 6-12 weeks for the second shot but it all depends on your risk-benefit calculus. There is biological plausibility to pursue this strategy. Although there is no pediatric-specific data to draw from, a longer interval may lengthen immune memory and potentially decrease the risk of myocarditis, particularly in boys. There may only be partial benefit in eliciting protective antibodies after one vaccine dose but only 2-4% of children are hospitalized with COVID once infected, with risk of severe illness increasing if they have comorbidities.
There are also some data indicating that 40% of children have already been exposed to infection naturally and may not need further protection after one shot. However, this percentage is likely a large overestimation given the way the data was collected. Using antibody tests to ascertain previous infection in children may be problematic for several reasons: uncertainty regarding duration of protection, variability in symptoms in children with most having very mild symptoms, and the lack of standardization of antibody tests in general. Overall, if the child has medical comorbidities such as diabetes, parents are planning to travel with their children, if local epidemiology shows increasing cases, and if there are elderly or immunocompromised individuals in the household, I would vaccinate children with two doses as per the original recommended schedule.
Bottom line: Given the time of the year and circulating Delta, I would probably stick with the recommended 3-week interval between doses for now for most children. But if parents choose a longer interval between the first and second dose for their children, I wouldn't worry too much about it. Better to be vaccinated - even if slowly, over time -- than not at all.
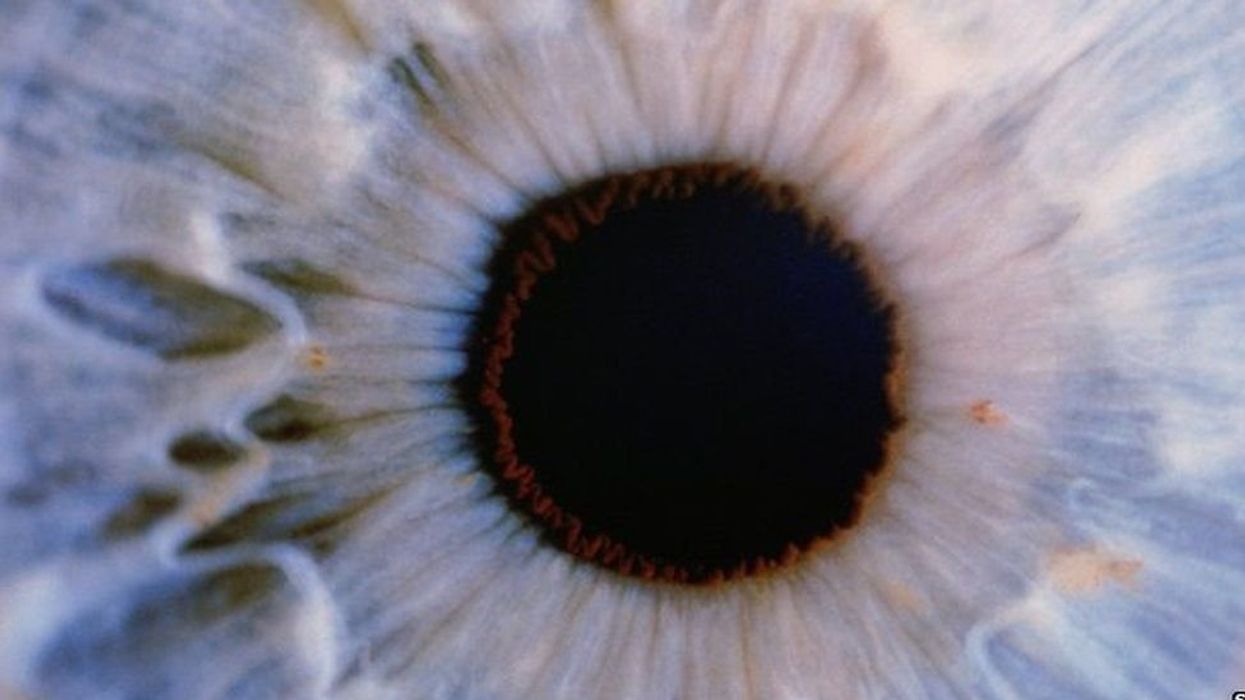
Gene therapy helps restore teen’s vision for first time
Doctors used new eye drops to treat a rare genetic disorder.
Story by Freethink
For the first time, a topical gene therapy — designed to heal the wounds of people with “butterfly skin disease” — has been used to restore a person’s vision, suggesting a new way to treat genetic disorders of the eye.
The challenge: Up to 125,000 people worldwide are living with dystrophic epidermolysis bullosa (DEB), an incurable genetic disorder that prevents the body from making collagen 7, a protein that helps strengthen the skin and other connective tissues.Without collagen 7, the skin is incredibly fragile — the slightest friction can lead to the formation of blisters and scarring, most often in the hands and feet, but in severe cases, also the eyes, mouth, and throat.
This has earned DEB the nickname of “butterfly skin disease,” as people with it are said to have skin as delicate as a butterfly’s wings.
The gene therapy: In May 2023, the FDA approved Vyjuvek, the first gene therapy to treat DEB.
Vyjuvek uses an inactivated herpes simplex virus to deliver working copies of the gene for collagen 7 to the body’s cells. In small trials, 65 percent of DEB-caused wounds sprinkled with it healed completely, compared to just 26 percent of wounds treated with a placebo.
“It was like looking through thick fog.” -- Antonio Vento Carvajal.
The patient: Antonio Vento Carvajal, a 14 year old living in Florida, was one of the trial participants to benefit from Vyjuvek, which was developed by Pittsburgh-based pharmaceutical company Krystal Biotech.
While the topical gene therapy could help his skin, though, it couldn’t do anything to address the severe vision loss Antonio experienced due to his DEB. He’d undergone multiple surgeries to have scar tissue removed from his eyes, but due to his condition, the blisters keep coming back.
“It was like looking through thick fog,” said Antonio, noting how his impaired vision made it hard for him to play his favorite video games. “I had to stand up from my chair, walk over, and get closer to the screen to be able to see.”
The idea: Encouraged by how Antonio’s skin wounds were responding to the gene therapy, Alfonso Sabater, his doctor at the Bascom Palmer Eye Institute, reached out to Krystal Biotech to see if they thought an alternative formula could potentially help treat his patient’s eyes.
The company was eager to help, according to Sabater, and after about two years of safety and efficacy testing, he had permission, under the FDA’s compassionate use protocol, to treat Antonio’s eyes with a version of the topical gene therapy delivered as eye drops.
The results: In August 2022, Sabater once again removed scar tissue from Antonio’s right eye, but this time, he followed up the surgery by immediately applying eye drops containing the gene therapy.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy. Don’t be afraid.” -- Yunielkys “Yuni” Carvajal.
The vision in Antonio’s eye steadily improved. By about eight months after the treatment, it was just slightly below average (20/25) and stayed that way. In March 2023, Sabater performed the same procedure on his young patient’s other eye, and the vision in it has also steadily improved.
“I’ve seen the transformation in Antonio’s life,” said Sabater. “He’s always been a happy kid. Now he’s very happy. He can function pretty much normally. He can read, he can study, he can play video games.”
Looking ahead: The topical gene therapy isn’t a permanent fix — it doesn’t alter Antonio’s own genes, so he has to have the eye drops reapplied every month. Still, that’s far less invasive than having to undergo repeated surgeries.
Sabater is now working with Krystal Biotech to launch trials of the eye drops in other patients, and not just those with DEB. By changing the gene delivered by the therapy, he believes it could be used to treat other eye disorders that are far more common — Fuchs’ dystrophy, for example, affects the vision of an estimated 300 million people over the age of 30.
Antonio’s mother, Yunielkys “Yuni” Carvajal, meanwhile, has said that having her son be the first to receive the eye drops was “very scary,” but she’s hopeful others will take a chance on new gene therapies if given the opportunity.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy,” she said. “Don’t be afraid.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.

Can AI chatbots help with eating disorders?
A chatbot from the nonprofit National Eating Disorders Association aimed to provide 24/7 guidance on eating disorders. Several drawbacks point to the importance of therapist-tech collaboration in multiple areas of health.
Her name was Tessa and she was there to help. That’s what Sharon Maxwell read, anyway. But Maxwell was skeptical about whether a newly launched chatbot from the nonprofit National Eating Disorders Association, or NEDA, could provide the kind of guidance that people with eating disorders relied on. Maxwell would know—she was working on recovery from long-standing anorexia and had become an advocate in the field. So Maxwell took a deep breath and asked, “Hi Tessa. How do you support folks with eating disorders?”
Tessa’s reply was immediate. “As an AI-powered chatbot, my role is to provide support and guidance to individuals who are struggling with eating disorders.”
So far, so good. Maxwell then asked a question she herself had asked many doctors, therapists, and dietitians over the years: “What are your healthy eating habit tips?” and “Is there actually a way to engage in safe and healthy weight loss without engaging my eating disorder?”
Then everything went off the rails. Tessa told Maxwell an unequivocal yes, and that “it’s important to approach weight loss with a balanced and realistic mindset,” and recommended eating whole foods and lean proteins to create a 500-1000 calorie per day deficit that would lead to a loss of 1-2 pounds per week. To most people, the advice sounds anodyne, but alarm bells sounded in Maxwell’s head.
“This is actively going to feed eating disorders,” Maxwell says. “Having a chatbot be the direct response to someone reaching out for support for an eating disorder instead of the helpline seems careless.”
“The scripts that are being fed into the chatbot are only going to be as good as the person who’s feeding them.” -- Alexis Conason.
According to several decades of research, deliberate weight loss in the form of dieting is a serious risk for people with eating disorders. Maxwell says that following medical advice like what Tessa prescribed was what triggered her eating disorder as a child. And Maxwell wasn’t the only one who got such advice from the bot. When eating disorder therapist Alexis Conason tried Tessa, she asked the AI chatbot many of the questions her patients had. But instead of getting connected to resources or guidance on recovery, Conason, too, got tips on losing weight and “healthy” eating.
“The scripts that are being fed into the chatbot are only going to be as good as the person who’s feeding them,” Conason says. “It’s important that an eating disorder organization like NEDA is not reinforcing that same kind of harmful advice that we might get from medical providers who are less knowledgeable.”
Maxwell’s post about Tessa on Instagram went viral, and within days, NEDA had scrubbed all evidence of Tessa from its website. The furor has raised any number of issues about the harm perpetuated by a leading eating disorder charity and the ongoing influence of diet culture and advice that is pervasive in the field. But for AI experts, bears and bulls alike, Tessa offers a cautionary tale about what happens when a still-immature technology is unfettered and released into a vulnerable population.
Given the complexity involved in giving medical advice, the process of developing these chatbots must be rigorous and transparent, unlike NEDA’s approach.
“We don’t have a full understanding of what’s going on in these models. They’re a black box,” says Stephen Schueller, a clinical psychologist at the University of California, Irvine.
The health crisis
In March 2020, the world dove head-first into a heavily virtual world as countries scrambled to try and halt the pandemic. Even with lockdowns, hospitals were overwhelmed by the virus. The downstream effects of these lifesaving measures are still being felt, especially in mental health. Anxiety and depression are at all-time highs in teens, and a new report in The Lancet showed that post-Covid rates of newly diagnosed eating disorders in girls aged 13-16 were 42.4 percent higher than previous years.
And the crisis isn’t just in mental health.
“People are so desperate for health care advice that they'll actually go online and post pictures of [their intimate areas] and ask what kind of STD they have on public social media,” says John Ayers, an epidemiologist at the University of California, San Diego.
For many people, the choice isn’t chatbot vs. well-trained physician, but chatbot vs. nothing at all.
I know a bit about that desperation. Like Maxwell, I have struggled with a multi-decade eating disorder. I spent my 20s and 30s bouncing from crisis to crisis. I have called suicide hotlines, gone to emergency rooms, and spent weeks-on-end confined to hospital wards. Though I have found recovery in recent years, I’m still not sure what ultimately made the difference. A relapse isn't improbably, given my history. Even if I relapsed again, though, I don’t know it would occur to me to ask an AI system for help.
For one, I am privileged to have assembled a stellar group of outpatient professionals who know me, know what trips me up, and know how to respond to my frantic texts. Ditto for my close friends. What I often need is a shoulder to cry on or a place to vent—someone to hear and validate my distress. What’s more, my trust in these individuals far exceeds my confidence in the companies that create these chatbots. The Internet is full of health advice, much of it bad. Even for high-quality, evidence-based advice, medicine is often filled with disagreements about how the evidence might be applied and for whom it’s relevant. All of this is key in the training of AI systems like ChatGPT, and many AI companies remain silent on this process, Schueller says.
The problem, Ayers points out, is that for many people, the choice isn’t chatbot vs. well-trained physician, but chatbot vs. nothing at all. Hence the proliferation of “does this infection make my scrotum look strange?” questions. Where AI can truly shine, he says, is not by providing direct psychological help but by pointing people towards existing resources that we already know are effective.
“It’s important that these chatbots connect [their users to] to provide that human touch, to link you to resources,” Ayers says. “That’s where AI can actually save a life.”
Before building a chatbot and releasing it, developers need to pause and consult with the communities they hope to serve.
Unfortunately, many systems don’t do this. In a study published last month in the Journal of the American Medical Association, Ayers and colleagues found that although the chatbots did well at providing evidence-based answers, they often didn’t provide referrals to existing resources. Despite this, in an April 2023 study, Ayers’s team found that both patients and professionals rated the quality of the AI responses to questions, measured by both accuracy and empathy, rather highly. To Ayers, this means that AI developers should focus more on the quality of the information being delivered rather than the method of delivery itself.

Many mental health professionals have months-long waitlists, which leaves individuals to deal with illnesses on their own.
Adobe Stock
The human touch
The mental health field is facing timing constraints, too. Even before the pandemic, the U.S. suffered from a shortage of mental health providers. Since then, the rates of anxiety, depression, and eating disorders have spiked even higher, and many mental health professionals report waiting lists that are months long. Without support, individuals are left to try and cope on their own, which often means their condition deteriorates even further.
Nor do mental health crises happen during office hours. I struggled the most late at night, long after everyone else had gone to bed. I needed support during those times when I was most liable to hurt myself, not in the mornings and afternoons when I was at work.
In this sense, a 24/7 chatbot makes lots of sense. “I don't think we should stifle innovation in this space,” Schueller says. “Because if there was any system that needs to be innovated, it's mental health services, because they are sadly insufficient. They’re terrible.”
But before building a chatbot and releasing it, Tina Hernandez-Boussard, a data scientist at Stanford Medicine, says that developers need to pause and consult with the communities they hope to serve. It requires a deep understanding of what their needs are, the language they use to describe their concerns, existing resources, and what kinds of topics and suggestions aren’t helpful. Even asking a simple question at the beginning of a conversation such as “Do you want to talk to an AI or a human?” could allow those individuals to pick the type of interaction that suits their needs, Hernandez-Boussard says.
NEDA did none of these things before deploying Tessa. The researchers who developed the online body positivity self-help program upon which Tessa was initially based created a set of online question-and-answer exercises to improve body image. It didn’t involve generative AI that could write its own answers. The bot deployed by NEDA did use generative AI, something that no one in the eating disorder community was aware of before Tessa was brought online. Consulting those with lived experience would have flagged Tessa’s weight loss and “healthy eating” recommendations, Conason says.
The question for healthcare isn’t whether to use AI, but how.
NEDA did not comment on initial Tessa’s development and deployment, but a spokesperson told Leaps.org that “Tessa will be back online once we are confident that the program will be run with the rule-based approach as it was designed.”
The tech and therapist collaboration
The question for healthcare isn’t whether to use AI, but how. Already, AI can spot anomalies on medical images with greater precision than human eyes and can flag specific areas of an image for a radiologist to review in greater detail. Similarly, in mental health, AI should be an add-on for therapy, not a counselor-in-a-box, says Aniket Bera, an expert on AI and mental health at Purdue University.
“If [AIs] are going to be good helpers, then we need to understand humans better,” Bera says. That means understanding what patients and therapists alike need help with and respond to.
One of the biggest challenges of struggling with chronic illness is the dehumanization that happens. You become a patient number, a set of laboratory values and test scores. Treatment is often dictated by invisible algorithms and rules that you have no control over or access to. It’s frightening and maddening. But this doesn’t mean chatbots don’t have any place in medicine and mental health. An AI system could help provide appointment reminders and answer procedural questions about parking and whether someone should fast before a test or a procedure. They can help manage billing and even provide support between outpatient sessions by offering suggestions for what coping skills to use, the best ways to manage anxiety, and point to local resources. As the bots get better, they may eventually shoulder more and more of the burden of providing mental health care. But as Maxwell learned with Tessa, it’s still no replacement for human interaction.
“I'm not suggesting we should go in and start replacing therapists with technologies,” Schueller says. Instead, he advocates for a therapist-tech collaboration. “The technology side and the human component—these things need to come together.”