Six Questions about the Kids' COVID Vaccine, Answered by an Infectious Disease Doctor

The author, an infectious disease physician, pictured with his two daughters who are getting vaccinated against COVID-19.
I enthusiastically support the vaccination against COVID for children aged 5-11 years old. As an infectious disease doctor who took care of hundreds of COVID-19 patients over the past 20 months, I have seen the immediate and long-term consequences of COVID-19 on patients – and on their families. As a father of two daughters, I have lived through the fear and anxiety of protecting my kids at all cost from the scourges of the pandemic and worried constantly about bringing the virus home from work.
It is imperative that we vaccinate as many children in the community as possible. There are several reasons why. First children do get sick from COVID-19. Over the course of the pandemic in the U.S, more than 2 million children aged 5-11 have become infected, more than 8000 have been hospitalized, and more than 100 have died, making COVID one of the top 10 causes of pediatric deaths in this age group over the past year. Children are also susceptible to chronic consequences of COVID such as long COVID and multisystem inflammatory syndrome in children (MIS-C). Most studies demonstrate that 10-30% of children will develop chronic symptoms following COVID-19. These include complaints of brain fog, fatigue, trouble breathing, fever, headache, muscle and joint pains, abdominal pain, mood swings and even psychiatric disorders. Symptoms typically last from 4-8 weeks in children, with some reporting symptoms that persist for many months.
Second, children are increasingly recognized as vectors who can bring infection into the house, potentially transmitting infection to vulnerable household members. Finally, we have all seen the mayhem that results when one child in the classroom becomes infected with COVID and the other students get sent home to quarantine – across the U.S., more than 2000 schools have been affected this way.
We now have an extraordinarily effective vaccine with more than 90 percent efficacy at preventing symptomatic infection. Vaccinating children will boost our countrywide vaccination rate which is trailing many countries after an early start. Nevertheless, there are still many questions and concerns that parents have as the vaccine gets rolled out. I will address six of them here.
"Novel Vaccine Technology"
Even though this is a relatively new vaccine, the technology is not new. Scientists had worked on mRNA vaccines for decades prior to the COVID mRNA vaccine breakthrough. Furthermore, experience with the Pfizer COVID vaccine is rapidly growing. By now it has been more than a year and a half since the Pfizer trials began in March 2020, and more than 7 billion doses have already been administered globally, including in 13.7 million adolescents in the U.S. alone.
"Will This Vaccine Alter My Child's DNA?"
No. This is not how mRNA works. DNA is present in the cell's nucleus. The mRNA only stays in the outside cytoplasm, gets destroyed and never enters the inner sanctum of the nucleus. Furthermore, for the mRNA to be ever integrated into DNA, it requires a special enzyme called reverse transcriptase which humans don't have. Proteins (that look like the spike proteins on SARS-CoV-2) are made directly from this mRNA message without involvement of our DNA at any time. Pieces of spike proteins get displayed on the outside of our cells and our body makes protective antibodies that then protects us handily against the future real virus if it were ever to enter our (or our children's) bodies. Our children's DNA or genes can never be affected by an mRNA vaccine.
"Lack of Info on Long-Term Side Effects"
Unlike medications that are taken daily or periodically and can build up over time, the mRNA in the Pfizer vaccine is evanescent. It literally is just the messenger (that is what the "m" in mRNA stands for) and the messenger quickly disappears. mRNA is extremely fragile and easily inactivated – that's why we need to encase it in a special fatty bubble and store the vaccines at extremely cold temperatures. Our cells break down and destroy the mRNA within a few days after receiving the instructions to make the virus spike proteins. The presence of these fragments of the virus (note this is not "live" virus) prompts our immune system to generate protective antibodies to the real thing. Our bodies break down mRNA all the time in normal cellular processes – this is nothing new.
What the transience of the delivery system means is that most of the effects of the mRNA vaccines are expected to be more immediate (sore arm, redness at the site, fever, chills etc.), with no long-term side effects anticipated. A severe allergic response has been reported to occur in some generally within the first 15 minutes, is very rare, and everyone gets observed for that as part of standard vaccine administration. Even with the very uncommon complication of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) seen primarily in young men under the age of 30 following mRNA vaccines, these typically happen within days to 2 weeks and many return to work or school in days. In the 70-year history of pediatric (and adult vaccines), dangerous complications happen in the first two months. There have been millions of adolescents as young as 12 years and thousands in the initial trial of children aged 5-11 who have already received the vaccine and are well beyond the two-month period of observation. There is no biological reason to believe that younger children will have a different long-term side effect profile compared to adolescents or adults.
"Small Sample Size in Kids and the Trial Design"
Although the Pfizer trial in children aged 5-11 was relatively small, it was big enough to give us statistical confidence in assessing safety and efficacy outcomes. Scientists spend a lot of time determining the right sample size of a study during the design phase. On one hand, you want to conduct the study efficiently so that resources are used in a cost-effective way and that you get a timely answer, especially in a fast-moving pandemic. On the other hand, you want to make sure you have enough sample size so that you can answer the question confidently as to whether the intervention works and whether there are adverse effects. The more profound the effect size of the intervention (in this case the vaccine), the fewer the numbers of children needed in the trials.
Statistics help investigators determine whether the results seen would have appeared by chance or not. In this case, the effect was real and impressive. Over 3,000 children around the world have received the vaccines through the trials alone with no serious side effects detected. The first press release reported that the immune response in children aged 5-11 was similar (at one-third the vaccine dose) to the response in the comparator group aged 16-25 years old. Extrapolating clinical efficacy results from immune response measurements ("immunobridging" study) would already have been acceptable if this was the only data. This is a standard trial design for many pediatric vaccines. Vaccines are first tested in the lab, followed by animals then adults. Only when deemed safe in adults and various regulatory bodies have signed off, do the pediatric vaccine trials commence.
Because children's immune systems and bodies are in a constant state of development, the vaccines must be right-sized. Investigators typically conduct "age de-escalation" studies in various age groups. The lowest dose is first tried so see if that is effective, then the dose is increased gradually as needed. Immune response is the easiest, safest and most efficient way to test the efficacy of pediatric vaccines. This is a typical size and design of a childhood vaccine seeking regulatory approval. There is no reason to think that the clinical efficacy would be any different in children vs. adults for a given antibody response, given the experience already in the remainder of the population, including older children and adolescents. Although this was primarily designed as an "immunobridging" study, the initial immunologic response data was followed by real clinical outcomes in this population. Reporting on the outcomes of 2,268 children in the randomized controlled trial, the vaccine was 90.7% effective at preventing symptomatic infection.
"Fear of Myocarditis"
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining of the heart) have been associated with receipt of the mRNA vaccines, particularly among male adolescents and young adults, typically within a few days after receiving the second dose. But this is very rare. For every million vaccine recipients, you would expect 41 cases in males, and 4 cases in females aged 12-29 years-old. The risk in older age groups is substantially lower. It is important to recognize that the risk of myocarditis associated with COVID is substantially higher. Patients present with new chest pain, shortness of breath, or palpitations after receiving an mRNA vaccine (more common after the second dose). But outcomes are good if associated with the vaccine. Most respond well to treatment and resolve symptoms within a week. There have been no deaths associated with vaccine-associated myocarditis.
In contrast, COVID-associated myocarditis has been associated with more severe cases as well as other complications including chronic symptoms of long COVID. The risk of myocarditis is likely related to vaccine dose, so the fact that one-third the dose of the vaccine will be used in the 5-11 year-olds is expected to correspond to a lower risk of myocarditis. At the lower dose given to younger kids, there has been a lower incidence of adverse effects reported compared to older children and adults who received the full dose. In addition, baseline rates of myocarditis not associated with vaccination are much lower in children ages 5-11 years than in older children, so the same may hold true for vaccine-associated myocarditis cases. This is because myocarditis is associated with sex hormones (particularly testosterone) that surge during puberty. In support of this, the incidence of vaccine-associated myocarditis is lower in 12–15-year-old boys, compared to those who were older than 16 years old. There were no cases of myocarditis reported in the experience to date of 5–11-year-old children in the trials, although the trial was too small to pick up on such a rare effect.
"Optimal Dose Spacing Interval: Longer Than 3 Weeks?"
There is a biologic basis for increasing the interval between vaccine doses in general. Priming the immune system with the first shot and then waiting gives the second shot a better chance of prompting a secondary immune reaction that results in a more durable response (with more T cell driven immune memory). One study from the U.K. showed that the antibody response in people over 80 was more than 3 times higher if they delayed the second dose to after 12 weeks for the Pfizer vaccine instead of the 3 weeks studied in trials. In a study of 503 British health care workers, there were twice as many neutralizing antibodies produced in a longer interval group (6-14 weeks) versus a shorter interval group (3-4 weeks) between doses. However, the safety and efficacy with longer intervals has not been evaluated in the pediatric or other COVID vaccine trials.
In the U.S., the C.D.C. reported that 88 percent of counties are at a "high" or "substantial" level of community transmission. Also, Europe is already experiencing a winter surge of infections that may predict more U.S. winter cases as international travel reopens. During a time of high community virus burden with a highly transmissible Delta variant, relying on one dose of vaccine for several more weeks until the second may leave many more susceptible to infection while waiting. One study from England showed that one dose of the Pfizer vaccine was only 33% protective against symptomatic Delta infection in contrast to 50% for the Alpha variant in adults. There has been no corollary information in children but we would expect less protection in general from one vaccine dose vs. two. This is a particularly important issue with the upcoming holiday season when an increased number of families will travel. Some countries such as the U.K. and Norway have proceeded with only offering older than 12 year-olds one dose of vaccine rather than two, but this was before the current European surge which may change the risk-benefit calculus. There are no plans to only offer one vaccine dose in the U.S. at this time. However a lower dose of the vaccine will likely be studied in the future for adolescents aged 12-15.
For parents worried about the potential risk of adverse effects of two doses of vaccines in their children, it is reasonable to wait 6-12 weeks for the second shot but it all depends on your risk-benefit calculus. There is biological plausibility to pursue this strategy. Although there is no pediatric-specific data to draw from, a longer interval may lengthen immune memory and potentially decrease the risk of myocarditis, particularly in boys. There may only be partial benefit in eliciting protective antibodies after one vaccine dose but only 2-4% of children are hospitalized with COVID once infected, with risk of severe illness increasing if they have comorbidities.
There are also some data indicating that 40% of children have already been exposed to infection naturally and may not need further protection after one shot. However, this percentage is likely a large overestimation given the way the data was collected. Using antibody tests to ascertain previous infection in children may be problematic for several reasons: uncertainty regarding duration of protection, variability in symptoms in children with most having very mild symptoms, and the lack of standardization of antibody tests in general. Overall, if the child has medical comorbidities such as diabetes, parents are planning to travel with their children, if local epidemiology shows increasing cases, and if there are elderly or immunocompromised individuals in the household, I would vaccinate children with two doses as per the original recommended schedule.
Bottom line: Given the time of the year and circulating Delta, I would probably stick with the recommended 3-week interval between doses for now for most children. But if parents choose a longer interval between the first and second dose for their children, I wouldn't worry too much about it. Better to be vaccinated - even if slowly, over time -- than not at all.
After spaceflight record, NASA looks to protect astronauts on even longer trips
NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

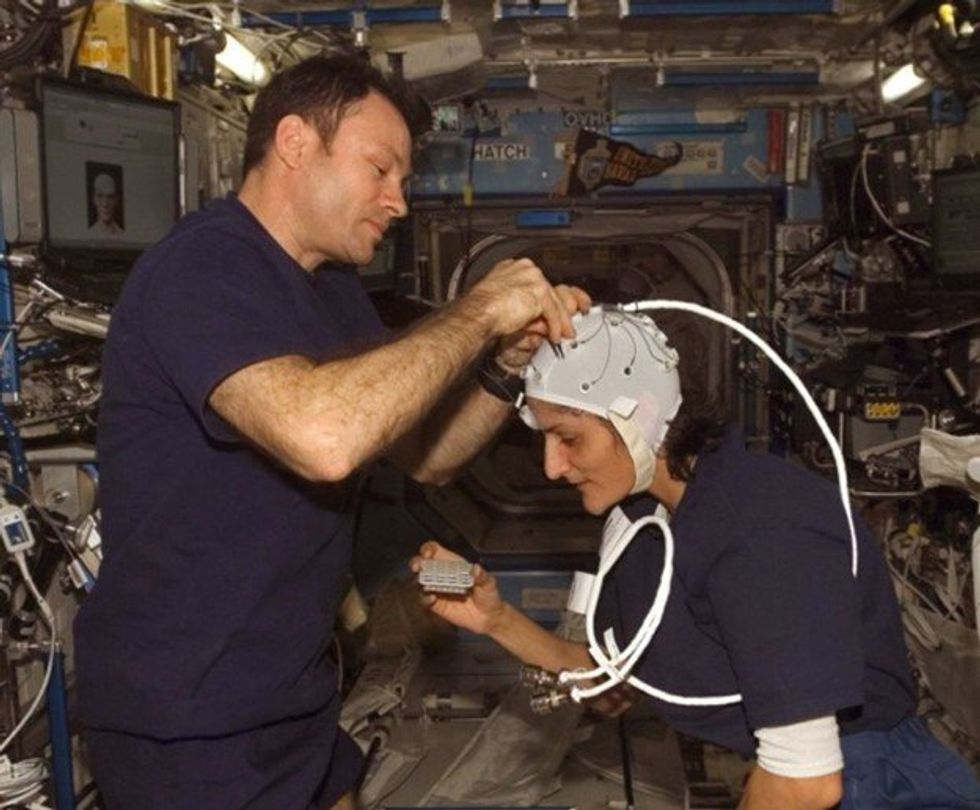
NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
A newly discovered brain cell may lead to better treatments for cognitive disorders
Swiss researchers have found a type of brain cell that appears to be a hybrid of the two other main types — and it could lead to new treatments for brain disorders.
Swiss researchers have discovered a third type of brain cell that appears to be a hybrid of the two other primary types — and it could lead to new treatments for many brain disorders.
The challenge: Most of the cells in the brain are either neurons or glial cells. While neurons use electrical and chemical signals to send messages to one another across small gaps called synapses, glial cells exist to support and protect neurons.
Astrocytes are a type of glial cell found near synapses. This close proximity to the place where brain signals are sent and received has led researchers to suspect that astrocytes might play an active role in the transmission of information inside the brain — a.k.a. “neurotransmission” — but no one has been able to prove the theory.
A new brain cell: Researchers at the Wyss Center for Bio and Neuroengineering and the University of Lausanne believe they’ve definitively proven that some astrocytes do actively participate in neurotransmission, making them a sort of hybrid of neurons and glial cells.
According to the researchers, this third type of brain cell, which they call a “glutamatergic astrocyte,” could offer a way to treat Alzheimer’s, Parkinson’s, and other disorders of the nervous system.
“Its discovery opens up immense research prospects,” said study co-director Andrea Volterra.
The study: Neurotransmission starts with a neuron releasing a chemical called a neurotransmitter, so the first thing the researchers did in their study was look at whether astrocytes can release the main neurotransmitter used by neurons: glutamate.
By analyzing astrocytes taken from the brains of mice, they discovered that certain astrocytes in the brain’s hippocampus did include the “molecular machinery” needed to excrete glutamate. They found evidence of the same machinery when they looked at datasets of human glial cells.
Finally, to demonstrate that these hybrid cells are actually playing a role in brain signaling, the researchers suppressed their ability to secrete glutamate in the brains of mice. This caused the rodents to experience memory problems.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Andrea Volterra, University of Lausanne.
But why? The researchers aren’t sure why the brain needs glutamatergic astrocytes when it already has neurons, but Volterra suspects the hybrid brain cells may help with the distribution of signals — a single astrocyte can be in contact with thousands of synapses.
“Often, we have neuronal information that needs to spread to larger ensembles, and neurons are not very good for the coordination of this,” researcher Ludovic Telley told New Scientist.
Looking ahead: More research is needed to see how the new brain cell functions in people, but the discovery that it plays a role in memory in mice suggests it might be a worthwhile target for Alzheimer’s disease treatments.
The researchers also found evidence during their study that the cell might play a role in brain circuits linked to seizures and voluntary movements, meaning it’s also a new lead in the hunt for better epilepsy and Parkinson’s treatments.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Volterra.