COVID Variants Are Like “a Thief Changing Clothes” – and Our Camera System Barely Exists

Being able to track variants of concern in real time is crucial to our ability to stay ahead of the virus.
Whether it's "natural selection" as Darwin called it, or it's "mutating" as the X-Men called it, living organisms change over time, developing thumbs or more efficient protein spikes, depending on the organism and the demands of its environment. The coronavirus that causes COVID-19, SARS-CoV-2, is not an exception, and now, after the virus has infected millions of people around the globe for more than a year, scientists are beginning to see those changes.
The notorious variants that have popped up include B.1.1.7, sometimes called the UK variant, as well as P.1 and B.1.351, which seem to have emerged in Brazil and South Africa respectively. As vaccinations are picking up pace, officials are warning that now
is not the time to become complacent or relax restrictions because the variants aren't well understood.
Some appear to be more transmissible, and deadlier, while others can evade the immune system's defenses better than earlier versions of the virus, potentially undermining the effectiveness of vaccines to some degree. Genomic surveillance, the process of sequencing the genetic code of the virus widely to observe changes and patterns, is a critical way that scientists can keep track of its evolution and work to understand how the variants might affect humans.
"It's like a thief changing clothes"
It's important to note that viruses mutate all the time. If there were funding and personnel to sequence the genome of every sample of the virus, scientists would see thousands of mutations. Not every variant deserves our attention. The vast majority of mutations are not important at all, but recognizing those that are is a crucial tool in getting and staying ahead of the virus. The work of sequencing, analyzing, observing patterns, and using public health tools as necessary is complicated and confusing to those without years of specialized training.
Jeremy Kamil, associate professor of microbiology and immunology at LSU Health Shreveport, in Louisiana, says that the variants developing are like a thief changing clothes. The thief goes in your house, steals your stuff, then leaves and puts on a different shirt and a wig, in the hopes you won't recognize them. Genomic surveillance catches the "thief" even in those different clothes.
One of the tricky things about variants is recognizing the point at which they move from interesting, to concerning at a local level, to dangerous in a larger context.
Understanding variants, both the uninteresting ones and the potentially concerning ones, gives public health officials and researchers at different levels a useful set of tools. Locally, knowing which variants are circulating in the community helps leaders know whether mask mandates and similar measures should be implemented or discontinued, or whether businesses and schools can open relatively safely.
There's more to it than observing new variants
Analysis is complex, particularly when it comes to understanding which variants are of concern. "So the question is always if a mutation becomes common, is that a random occurrence?" says Phoebe Lostroh, associate professor of molecular biology at Colorado College. "Or is the variant the result of some kind of selection because the mutation changes some property about the virus that makes it reproduce more quickly than variants of the virus that don't have that mutation? For a virus, [mutations can affect outcomes like] how much it replicates inside a person's body, how much somebody breathes it out, whether the particles that somebody might breathe in get smaller and can lead to greater transmission."
Along with all of those factors, accurate and useful genomic surveillance requires an understanding of where variants are occurring, how they are related, and an examination of why they might be prevalent.
For example, if a potentially worrisome variant appears in a community and begins to spread very quickly, it's not time to raise a public health alarm until several important questions have been answered, such as whether the variant is spreading due to specific events, or if it's happening because the mutation has allowed the virus to infect people more efficiently. Kamil offered a hypothetical scenario to explain: Imagine that a member of a community became infected and the virus mutated. That person went to church and three more people were infected, but one of them went to a karaoke bar and while singing infected 100 other people. Examining the conditions under which the virus has spread is, therefore, an essential part of untangling whether a mutation itself made the virus more transmissible or if an infected person's behaviors contributed to a local outbreak.
One of the tricky things about variants is recognizing the point at which they move from interesting, to concerning at a local level, to dangerous in a larger context. Genomic sequencing can help with that, but only when it's coordinated. When the same mutation occurs frequently, but is localized to one region, it's a concern, but when the same mutation happens in different places at the same time, it's much more likely that the "virus is learning that's a good mutation," explains Kamil.
The process is called convergent evolution, and it was a fascinating topic long before COVID. Just as your heritage can be traced through DNA, so can that of viruses, and when separate lineages develop similar traits it's almost like scientists can see evolution happening in real time. A mutation to SARS-CoV-2 that happens in more than one place at once is a mutation that makes it easier in some way for the virus to survive and that is when it may become alarming. The widespread, documented variants P.1 and B.1.351 are examples of convergence because they share some of the same virulent mutations despite having developed thousands of miles apart.
However, even variants that are emerging in different places at the same time don't present the kind of threat SARS-CoV-2 did in 2019. "This is nature," says Kamil. "It just means that this virus will not easily be driven to extinction or complete elimination by vaccines." Although a person who has already had COVID-19 can be reinfected with a variant, "it is almost always much milder disease" than the original infection, Kamil adds. Rather than causing full-fledged disease, variants have the potiental to "penetrate herd immunity, spreading relatively quietly among people who have developed natural immunity or been vaccinated, until the virus finds someone who has no immunity yet, and that person would be at risk of hospitalization-grade severe disease or death."
Surveillance and predictions
According to Lostroh, genomic surveillance can help scientists predict what's going to happen. "With the British strain, for instance, that's more transmissible, you can measure how fast it's doubling in the population and you can sort of tell whether we should take more measures against this mutation. Should we shut things down a little longer because that mutation is present in the population? That could be really useful if you did enough sampling in the population that you knew where it was," says Lostroh. If, for example, the more transmissible strain was present in 50 percent of cases, but in another county or state it was barely present, it would allow for rolling lockdowns instead of sweeping measures.
Variants are also extremely important when it comes to the development, manufacture, and distribution of vaccines. "You're also looking at medical countermeasures, such as whether your vaccine is still effective, or if your antiviral needs to be updated," says Lane Warmbrod, a senior analyst and research associate at Johns Hopkins Center for Health Security.
Properly funded and extensive genomic surveillance could eventually help control endemic diseases, too, like the seasonal flu, or other common respiratory infections. Kamil says he envisions a future in which genomic surveillance allows for prediction of sickness just as the weather is predicted today. "It's a 51 for infection today at the San Francisco Airport. There's been detection of some respiratory viruses," he says, offering an example. He says that if you're a vulnerable person, if you're immune-suppressed for some reason, you may want to wear a mask based on the sickness report.
The U.S. has the ability, but lacks standards
The benefits of widespread genomic surveillance are clear, and the United States certainly has the necessary technology, equipment, and personnel to carry it out. But, it's not happening at the speed and extent it needs to for the country to gain the benefits.
"The numbers are improving," said Kamil. "We're probably still at less than half a percent of all the samples that have been taken have been sequenced since the beginning of the pandemic."
Although there's no consensus on how many sequences is ideal for a robust surveillance program, modeling performed by the company Illumina suggests about 5 percent of positive tests should be sequenced. The reasons the U.S. has lagged in implementing a sequencing program are complex and varied, but solvable.
Perhaps the most important element that is currently missing is leadership. In order to conduct an effective genomic surveillance program, there need to be standards. The Johns Hopkins Center for Health Security recently published a paper with recommendations as to what kinds of elements need to be standardized in order to make the best use of sequencing technology and analysis.
"Along with which bioinformatic pipelines you're going to use to do the analyses, which sequencing strategy protocol are you going to use, what's your sampling strategy going to be, how is the data is going to be reported, what data gets reported," says Warmbrod. Currently, there's no guidance from the CDC on any of those things. So, while scientists can collect and report information, they may be collecting and reporting different information that isn't comparable, making it less useful for public health measures and vaccine updates.
Globally, one of the most important tools in making the information from genomic surveillance useful is GISAID, a platform designed for scientists to share -- and, importantly, to be credited for -- their data regarding genetic sequences of influenza. Originally, it was launched as a database of bird flu sequences, but has evolved to become an essential tool used by the WHO to make flu vaccine virus recommendations each year. Scientists who share their credentials have free access to the database, and anyone who uses information from the database must credit the scientist who uploaded that information.
Safety, logistics, and funding matter
Scientists at university labs and other small organizations have been uploading sequences to GISAID almost from the beginning of the pandemic, but their funding is generally limited, and there are no standards regarding information collection or reporting. Private, for-profit labs haven't had motivation to set up sequencing programs, although many of them have the logistical capabilities and funding to do so. Public health departments are understaffed, underfunded, and overwhelmed.
University labs may also be limited by safety concerns. The SARS-CoV-2 virus is dangerous, and there's a question of how samples should be transported to labs for sequencing.
Larger, for-profit organizations often have the tools and distribution capabilities to safely collect and sequence samples, but there hasn't been a profit motive. Genomic sequencing is less expensive now than ever before, but even at $100 per sample, the cost adds up -- not to mention the cost of employing a scientist with the proper credentials to analyze the sequence.
The path forward
The recently passed COVID-19 relief bill does have some funding to address genomic sequencing. Specifically, the American Rescue Plan Act includes $1.75 billion in funding for the Centers for Disease Control and Prevention's Advanced Molecular Detection (AMD) program. In an interview last month, CDC Director Rochelle Walensky said that the additional funding will be "a dial. And we're going to need to dial it up." AMD has already announced a collaboration called the Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance (SPHERES) Initiative that will bring together scientists from public health, academic, clinical, and non-profit laboratories across the country with the goal of accelerating sequencing.
Such a collaboration is a step toward following the recommendations in the paper Warmbrod coauthored. Building capacity now, creating a network of labs, and standardizing procedures will mean improved health in the future. "I want to be optimistic," she says. "The good news is there are a lot of passionate, smart, capable people who are continuing to work with government and work with different stakeholders." She cautions, however, that without a national strategy we won't succeed.
"If we maximize the potential and create that framework now, we can also use it for endemic diseases," she says. "It's a very helpful system for more than COVID if we're smart in how we plan it."
Out of Thin Air: A Fresh Solution to Farming’s Water Shortages
Dry, arid and remote farming regions are vulnerable to water shortages, but scientists are working on a promising new solution.
California has been plagued by perilous droughts for decades. Freshwater shortages have sparked raging wildfires and killed fruit and vegetable crops. And California is not alone in its danger of running out of water for farming; parts of the Southwest, including Texas, are battling severe drought conditions, according to the North American Drought Monitor. These two states account for 316,900 of the 2 million total U.S. farms.
But even as farming becomes more vulnerable due to water shortages, the world's demand for food is projected to increase 70 percent by 2050, according to Guihua Yu, an associate professor of materials science at The University of Texas at Austin.
"Water is the most limiting natural resource for agricultural production because of the freshwater shortage and enormous water consumption needed for irrigation," Yu said.
As scientists have searched for solutions, an alternative water supply has been hiding in plain sight: Water vapor in the atmosphere. It is abundant, available, and endlessly renewable, just waiting for the moment that technological innovation and necessity converged to make it fit for use. Now, new super-moisture-absorbent gels developed by Yu and a team of researchers can pull that moisture from the air and bring it into soil, potentially expanding the map of farmable land around the globe to dry and remote regions that suffer from water shortages.
"This opens up opportunities to turn those previously poor-quality or inhospitable lands to become useable and without need of centralized water and power supplies," Yu said.
A renewable source of freshwater
The hydrogels are a gelatin-like substance made from synthetic materials. The gels activate in cooler, humid overnight periods and draw water from the air. During a four-week experiment, Yu's team observed that soil with these gels provided enough water to support seed germination and plant growth without an additional liquid water supply. And the soil was able to maintain the moist environment for more than a month, according to Yu.
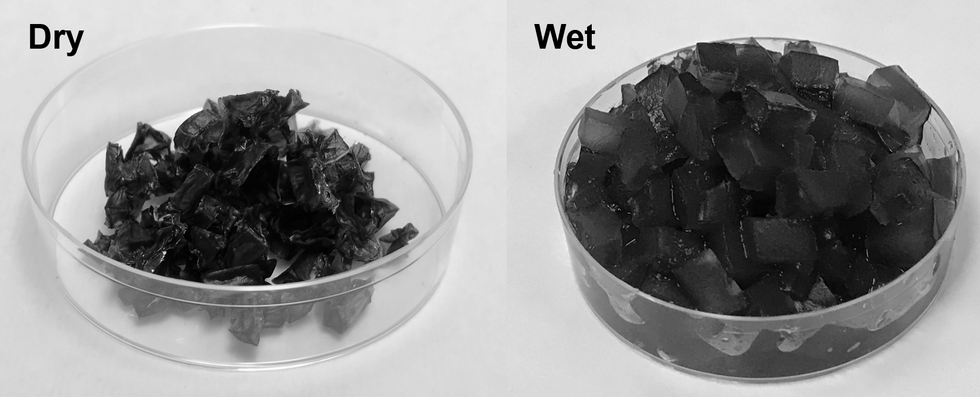
The super absorbent gels developed at the University of Texas at Austin.
Xingyi Zhou, UT Austin
"It is promising to liberate underdeveloped and drought areas from the long-distance water and power supplies for agricultural production," Yu said.
Crops also rely on fertilizer to maintain soil fertility and increase the production yield, but it is easily lost through leaching. Runoff increases agricultural costs and contributes to environmental pollution. The interaction between the gels and agrochemicals offer slow and controlled fertilizer release to maintain the balance between the root of the plant and the soil.
The possibilities are endless
Harvesting atmospheric water is exciting on multiple fronts. The super-moisture-absorbent gel can also be used for passively cooling solar panels. Solar radiation is the magic behind the process. Overnight, as temperatures cool, the gels absorb water hanging in the atmosphere. The moisture is stored inside the gels until the thermometer rises. Heat from the sun serves as the faucet that turns the gels on so they can release the stored water and cool down the panels. Effective cooling of the solar panels is important for sustainable long-term power generation.
In addition to agricultural uses and cooling for energy devices, atmospheric water harvesting technologies could even reach people's homes.
"They could be developed to enable easy access to drinking water through individual systems for household usage," Yu said.
Next steps
Yu and the team are now focused on affordability and developing practical applications for use. The goal is to optimize the gel materials to achieve higher levels of water uptake from the atmosphere.
"We are exploring different kinds of polymers and solar absorbers while exploring low-cost raw materials for production," Yu said.
The ability to transform atmospheric water vapor into a cheap and plentiful water source would be a game-changer. One day in the not-too-distant future, if climate change intensifies and droughts worsen, this innovation may become vital to our very survival.
On left, people excitedly line up for Salk's polio vaccine in 1957; on right, Joe Biden gets one of the COVID vaccines on December 21, 2020.
On the morning of April 12, 1955, newsrooms across the United States inked headlines onto newsprint: the Salk Polio vaccine was "safe, effective, and potent." This was long-awaited news. Americans had limped through decades of fear, unaware of what caused polio or how to cure it, faced with the disease's terrifying, visible power to paralyze and kill, particularly children.
The announcement of the polio vaccine was celebrated with noisy jubilation: church bells rang, factory whistles sounded, people wept in the streets. Within weeks, mass inoculation began as the nation put its faith in a vaccine that would end polio.
Today, most of us are blissfully ignorant of child polio deaths, making it easier to believe that we have not personally benefited from the development of vaccines. According to Dr. Steven Pinker, cognitive psychologist and author of the bestselling book Enlightenment Now, we've become blasé to the gifts of science. "The default expectation is not that disease is part of life and science is a godsend, but that health is the default, and any disease is some outrage," he says.
We're now in the early stages of another vaccine rollout, one we hope will end the ravages of the COVID-19 pandemic. And yet, the Pfizer, Moderna, and AstraZeneca vaccines are met with far greater hesitancy and skepticism than the polio vaccine was in the 50s.
In 2021, concerns over the speed and safety of vaccine development and technology plague this heroic global effort, but the roots of vaccine hesitancy run far deeper. Vaccine hesitancy has always existed in the U.S., even in the polio era, motivated in part by fears around "living virus" in a bad batch of vaccines produced by Cutter Laboratories in 1955. But in the last half century, we've witnessed seismic cultural shifts—loss of public trust, a rise in misinformation, heightened racial and socioeconomic inequality, and political polarization have all intensified vaccine-related fears and resistance. Making sense of how we got here may help us understand how to move forward.
The Rise and Fall of Public Trust
When the polio vaccine was released in 1955, "we were nearing an all-time high point in public trust," says Matt Baum, Harvard Kennedy School professor and lead author of several reports measuring public trust and vaccine confidence. Baum explains that the U.S. was experiencing a post-war boom following the Allied triumph in WWII, a popular Roosevelt presidency, and the rapid innovation that elevated the country to an international superpower.
The 1950s witnessed the emergence of nuclear technology, a space program, and unprecedented medical breakthroughs, adds Emily Brunson, Texas State University anthropologist and co-chair of the Working Group on Readying Populations for COVID-19 Vaccine. "Antibiotics were a game changer," she states. While before, people got sick with pneumonia for a month, suddenly they had access to pills that accelerated recovery.
During this period, science seemed to hold all the answers; people embraced the idea that we could "come to know the world with an absolute truth," Brunson explains. Doctors were portrayed as unquestioned gods, so Americans were primed to trust experts who told them the polio vaccine was safe.
"The emotional tone of the news has gone downward since the 1940s, and journalists consider it a professional responsibility to cover the negative."
That blind acceptance eroded in the 1960s and 70s as people came to understand that science can be inherently political. "Getting to an absolute truth works out great for white men, but these things affect people socially in radically different ways," Brunson says. As the culture began questioning the white, patriarchal biases of science, doctors lost their god-like status and experts were pushed off their pedestals. This trend continues with greater intensity today, as President Trump has led a campaign against experts and waged a war on science that began long before the pandemic.
The Shift in How We Consume Information
In the 1950s, the media created an informational consensus. The fundamental ideas the public consumed about the state of the world were unified. "People argued about the best solutions, but didn't fundamentally disagree on the factual baseline," says Baum. Indeed, the messaging around the polio vaccine was centralized and consistent, led by President Roosevelt's successful March of Dimes crusade. People of lower socioeconomic status with limited access to this information were less likely to have confidence in the vaccine, but most people consumed media that assured them of the vaccine's safety and mobilized them to receive it.
Today, the information we consume is no longer centralized—in fact, just the opposite. "When you take that away, it's hard for people to know what to trust and what not to trust," Baum explains. We've witnessed an increase in polarization and the technology that makes it easier to give people what they want to hear, reinforcing the human tendencies to vilify the other side and reinforce our preexisting ideas. When information is engineered to further an agenda, each choice and risk calculation made while navigating the COVID-19 pandemic is deeply politicized.
This polarization maps onto a rise in socioeconomic inequality and economic uncertainty. These factors, associated with a sense of lost control, prime people to embrace misinformation, explains Baum, especially when the situation is difficult to comprehend. "The beauty of conspiratorial thinking is that it provides answers to all these questions," he says. Today's insidious fragmentation of news media accelerates the circulation of mis- and disinformation, reaching more people faster, regardless of veracity or motivation. In the case of vaccines, skepticism around their origin, safety, and motivation is intensified.
Alongside the rise in polarization, Pinker says "the emotional tone of the news has gone downward since the 1940s, and journalists consider it a professional responsibility to cover the negative." Relentless focus on everything that goes wrong further erodes public trust and paints a picture of the world getting worse. "Life saved is not a news story," says Pinker, but perhaps it should be, he continues. "If people were more aware of how much better life was generally, they might be more receptive to improvements that will continue to make life better. These improvements don't happen by themselves."
The Future Depends on Vaccine Confidence
So far, the U.S. has been unable to mitigate the catastrophic effects of the pandemic through social distancing, testing, and contact tracing. President Trump has downplayed the effects and threat of the virus, censored experts and scientists, given up on containing the spread, and mobilized his base to protest masks. The Trump Administration failed to devise a national plan, so our national plan has defaulted to hoping for the "miracle" of a vaccine. And they are "something of a miracle," Pinker says, describing vaccines as "the most benevolent invention in the history of our species." In record-breaking time, three vaccines have arrived. But their impact will be weakened unless we achieve mass vaccination. As Brunson notes, "The technology isn't the fix; it's people taking the technology."
Significant challenges remain, including facilitating widespread access and supporting on-the-ground efforts to allay concerns and build trust with specific populations with historic reasons for distrust, says Brunson. Baum predicts continuing delays as well as deaths from other causes that will be linked to the vaccine.
Still, there's every reason for hope. The new administration "has its eyes wide open to these challenges. These are the kind of problems that are amenable to policy solutions if we have the will," Baum says. He forecasts widespread vaccination by late summer and a bounce back from the economic damage, a "Good News Story" that will bolster vaccine acceptance in the future. And Pinker reminds us that science, medicine, and public health have greatly extended our lives in the last few decades, a trend that can only continue if we're willing to roll up our sleeves.

