COVID Variants Are Like “a Thief Changing Clothes” – and Our Camera System Barely Exists

Being able to track variants of concern in real time is crucial to our ability to stay ahead of the virus.
Whether it's "natural selection" as Darwin called it, or it's "mutating" as the X-Men called it, living organisms change over time, developing thumbs or more efficient protein spikes, depending on the organism and the demands of its environment. The coronavirus that causes COVID-19, SARS-CoV-2, is not an exception, and now, after the virus has infected millions of people around the globe for more than a year, scientists are beginning to see those changes.
The notorious variants that have popped up include B.1.1.7, sometimes called the UK variant, as well as P.1 and B.1.351, which seem to have emerged in Brazil and South Africa respectively. As vaccinations are picking up pace, officials are warning that now
is not the time to become complacent or relax restrictions because the variants aren't well understood.
Some appear to be more transmissible, and deadlier, while others can evade the immune system's defenses better than earlier versions of the virus, potentially undermining the effectiveness of vaccines to some degree. Genomic surveillance, the process of sequencing the genetic code of the virus widely to observe changes and patterns, is a critical way that scientists can keep track of its evolution and work to understand how the variants might affect humans.
"It's like a thief changing clothes"
It's important to note that viruses mutate all the time. If there were funding and personnel to sequence the genome of every sample of the virus, scientists would see thousands of mutations. Not every variant deserves our attention. The vast majority of mutations are not important at all, but recognizing those that are is a crucial tool in getting and staying ahead of the virus. The work of sequencing, analyzing, observing patterns, and using public health tools as necessary is complicated and confusing to those without years of specialized training.
Jeremy Kamil, associate professor of microbiology and immunology at LSU Health Shreveport, in Louisiana, says that the variants developing are like a thief changing clothes. The thief goes in your house, steals your stuff, then leaves and puts on a different shirt and a wig, in the hopes you won't recognize them. Genomic surveillance catches the "thief" even in those different clothes.
One of the tricky things about variants is recognizing the point at which they move from interesting, to concerning at a local level, to dangerous in a larger context.
Understanding variants, both the uninteresting ones and the potentially concerning ones, gives public health officials and researchers at different levels a useful set of tools. Locally, knowing which variants are circulating in the community helps leaders know whether mask mandates and similar measures should be implemented or discontinued, or whether businesses and schools can open relatively safely.
There's more to it than observing new variants
Analysis is complex, particularly when it comes to understanding which variants are of concern. "So the question is always if a mutation becomes common, is that a random occurrence?" says Phoebe Lostroh, associate professor of molecular biology at Colorado College. "Or is the variant the result of some kind of selection because the mutation changes some property about the virus that makes it reproduce more quickly than variants of the virus that don't have that mutation? For a virus, [mutations can affect outcomes like] how much it replicates inside a person's body, how much somebody breathes it out, whether the particles that somebody might breathe in get smaller and can lead to greater transmission."
Along with all of those factors, accurate and useful genomic surveillance requires an understanding of where variants are occurring, how they are related, and an examination of why they might be prevalent.
For example, if a potentially worrisome variant appears in a community and begins to spread very quickly, it's not time to raise a public health alarm until several important questions have been answered, such as whether the variant is spreading due to specific events, or if it's happening because the mutation has allowed the virus to infect people more efficiently. Kamil offered a hypothetical scenario to explain: Imagine that a member of a community became infected and the virus mutated. That person went to church and three more people were infected, but one of them went to a karaoke bar and while singing infected 100 other people. Examining the conditions under which the virus has spread is, therefore, an essential part of untangling whether a mutation itself made the virus more transmissible or if an infected person's behaviors contributed to a local outbreak.
One of the tricky things about variants is recognizing the point at which they move from interesting, to concerning at a local level, to dangerous in a larger context. Genomic sequencing can help with that, but only when it's coordinated. When the same mutation occurs frequently, but is localized to one region, it's a concern, but when the same mutation happens in different places at the same time, it's much more likely that the "virus is learning that's a good mutation," explains Kamil.
The process is called convergent evolution, and it was a fascinating topic long before COVID. Just as your heritage can be traced through DNA, so can that of viruses, and when separate lineages develop similar traits it's almost like scientists can see evolution happening in real time. A mutation to SARS-CoV-2 that happens in more than one place at once is a mutation that makes it easier in some way for the virus to survive and that is when it may become alarming. The widespread, documented variants P.1 and B.1.351 are examples of convergence because they share some of the same virulent mutations despite having developed thousands of miles apart.
However, even variants that are emerging in different places at the same time don't present the kind of threat SARS-CoV-2 did in 2019. "This is nature," says Kamil. "It just means that this virus will not easily be driven to extinction or complete elimination by vaccines." Although a person who has already had COVID-19 can be reinfected with a variant, "it is almost always much milder disease" than the original infection, Kamil adds. Rather than causing full-fledged disease, variants have the potiental to "penetrate herd immunity, spreading relatively quietly among people who have developed natural immunity or been vaccinated, until the virus finds someone who has no immunity yet, and that person would be at risk of hospitalization-grade severe disease or death."
Surveillance and predictions
According to Lostroh, genomic surveillance can help scientists predict what's going to happen. "With the British strain, for instance, that's more transmissible, you can measure how fast it's doubling in the population and you can sort of tell whether we should take more measures against this mutation. Should we shut things down a little longer because that mutation is present in the population? That could be really useful if you did enough sampling in the population that you knew where it was," says Lostroh. If, for example, the more transmissible strain was present in 50 percent of cases, but in another county or state it was barely present, it would allow for rolling lockdowns instead of sweeping measures.
Variants are also extremely important when it comes to the development, manufacture, and distribution of vaccines. "You're also looking at medical countermeasures, such as whether your vaccine is still effective, or if your antiviral needs to be updated," says Lane Warmbrod, a senior analyst and research associate at Johns Hopkins Center for Health Security.
Properly funded and extensive genomic surveillance could eventually help control endemic diseases, too, like the seasonal flu, or other common respiratory infections. Kamil says he envisions a future in which genomic surveillance allows for prediction of sickness just as the weather is predicted today. "It's a 51 for infection today at the San Francisco Airport. There's been detection of some respiratory viruses," he says, offering an example. He says that if you're a vulnerable person, if you're immune-suppressed for some reason, you may want to wear a mask based on the sickness report.
The U.S. has the ability, but lacks standards
The benefits of widespread genomic surveillance are clear, and the United States certainly has the necessary technology, equipment, and personnel to carry it out. But, it's not happening at the speed and extent it needs to for the country to gain the benefits.
"The numbers are improving," said Kamil. "We're probably still at less than half a percent of all the samples that have been taken have been sequenced since the beginning of the pandemic."
Although there's no consensus on how many sequences is ideal for a robust surveillance program, modeling performed by the company Illumina suggests about 5 percent of positive tests should be sequenced. The reasons the U.S. has lagged in implementing a sequencing program are complex and varied, but solvable.
Perhaps the most important element that is currently missing is leadership. In order to conduct an effective genomic surveillance program, there need to be standards. The Johns Hopkins Center for Health Security recently published a paper with recommendations as to what kinds of elements need to be standardized in order to make the best use of sequencing technology and analysis.
"Along with which bioinformatic pipelines you're going to use to do the analyses, which sequencing strategy protocol are you going to use, what's your sampling strategy going to be, how is the data is going to be reported, what data gets reported," says Warmbrod. Currently, there's no guidance from the CDC on any of those things. So, while scientists can collect and report information, they may be collecting and reporting different information that isn't comparable, making it less useful for public health measures and vaccine updates.
Globally, one of the most important tools in making the information from genomic surveillance useful is GISAID, a platform designed for scientists to share -- and, importantly, to be credited for -- their data regarding genetic sequences of influenza. Originally, it was launched as a database of bird flu sequences, but has evolved to become an essential tool used by the WHO to make flu vaccine virus recommendations each year. Scientists who share their credentials have free access to the database, and anyone who uses information from the database must credit the scientist who uploaded that information.
Safety, logistics, and funding matter
Scientists at university labs and other small organizations have been uploading sequences to GISAID almost from the beginning of the pandemic, but their funding is generally limited, and there are no standards regarding information collection or reporting. Private, for-profit labs haven't had motivation to set up sequencing programs, although many of them have the logistical capabilities and funding to do so. Public health departments are understaffed, underfunded, and overwhelmed.
University labs may also be limited by safety concerns. The SARS-CoV-2 virus is dangerous, and there's a question of how samples should be transported to labs for sequencing.
Larger, for-profit organizations often have the tools and distribution capabilities to safely collect and sequence samples, but there hasn't been a profit motive. Genomic sequencing is less expensive now than ever before, but even at $100 per sample, the cost adds up -- not to mention the cost of employing a scientist with the proper credentials to analyze the sequence.
The path forward
The recently passed COVID-19 relief bill does have some funding to address genomic sequencing. Specifically, the American Rescue Plan Act includes $1.75 billion in funding for the Centers for Disease Control and Prevention's Advanced Molecular Detection (AMD) program. In an interview last month, CDC Director Rochelle Walensky said that the additional funding will be "a dial. And we're going to need to dial it up." AMD has already announced a collaboration called the Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance (SPHERES) Initiative that will bring together scientists from public health, academic, clinical, and non-profit laboratories across the country with the goal of accelerating sequencing.
Such a collaboration is a step toward following the recommendations in the paper Warmbrod coauthored. Building capacity now, creating a network of labs, and standardizing procedures will mean improved health in the future. "I want to be optimistic," she says. "The good news is there are a lot of passionate, smart, capable people who are continuing to work with government and work with different stakeholders." She cautions, however, that without a national strategy we won't succeed.
"If we maximize the potential and create that framework now, we can also use it for endemic diseases," she says. "It's a very helpful system for more than COVID if we're smart in how we plan it."
How One University Is Successfully Tackling COVID-19
Alma Mater, a beloved bronze mother figure on the campus of the University of Illinois, Champaign-Urbana, wears a mask to encourage students to do the same as they return for the fall semester. Students are also expected to take a COVID-19 test twice a week.
China, South Korea and other places controlled the SARS-CoV-2 epidemic with the early use of strict lockdown and aggressive electronic contact tracing, monitoring, and enforcement.
The tussles in America over voluntary social distancing and wearing a mask in public suggest that more stringent enforcement methods adopted elsewhere would not work here. But one American university has emerged as a model of tough love pandemic management.
While many universities have become hot spots of COVID-19 infections this fall when students returned to campus, the University of Illinois was an exception. It has gotten the virus under control, at least for the moment, at a rate that is far below the national average and with minimal social disruption. Can the program they implemented work in our broader society?
The Illinois model is a comprehensive one which, as elsewhere, includes masking and social distancing, but it also requires a twice-weekly saliva test for SARS-CoV-2. All students and employees are assigned test days when they swipe their ID card and spit in a plastic tube, which is collected hourly and taken to a campus lab.
There a simplified but highly sensitive PCR genetic test goes through many cycles of amplifying the viral RNA. "Tracking three different viral RNA [genes] gives us very high accuracy," explains Martin Burke, the professor who developed the system and is monitoring its implementation at the University of Illinois Urbana-Champaign. They immediately retest any positive sample to confirm the results, "So we think our false positive rate is extremely low. … The goal is to notify the positive person within 30 minutes of a positive test results becoming known."
Testing everyone so frequently, with a sensitive test that can quickly detect small amounts of the virus soon after infection, and isolating those who test positive before the virus can grow to volumes that make it very infectious helps the Illinois system break the chain of transmission.
"The testing we have done is not a silver bullet, it has to be done in combination with other mitigation measures. Our modeling shows that if you have masks, social distancing, and contact tracing you get a very dramatic, in fact synergistic effect with this combination,' says Burke. "So it really has to be a holistic approach with lots of community engagement in order to make this process successful."
The real teeth of enforcement are that people have to display their health status to gain access to campus facilities. A green check mark over their photo on a college ID phone app means they are good to go but a big red X means they are not current on their testing or have tested positive for the virus. Their ID is inactivated and they cannot enter campus facilities until they become compliant. Burke puts it bluntly; "We stop them from going where they want to go, a measure first used successfully with the pandemic in Wuhan, China.
He says they have learned from their experience and evolved their approach. "We never modeled for people who tested positive to ignore that result and go to or host parties, which could spread the infection." But several students did just that, and a few have been suspended for it.
So the university clamped down on enforcing isolation and now requires some higher risk persons to test three times a week to catch any infections earlier. Since more than 95 percent of new infections were among undergraduates, with no crossover from them to the local community, faculty, or graduate students, they have cut back testing of the latter two groups to just once a week.
About a thousand positive tests results have come back so far but no one has been hospitalized. Part of that likely is because the undergraduate population is largely young and healthy with few risk cofactors. But it may also be that with early identification and isolation, about five percent of dorm rooms have been set aside for that purpose, the person adopts healthier patterns of sleeping and eating that allows the immune system to better fight off the virus.
"But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
The logistics are quite impressive for the campus that in ordinary times is home to more than 50,000 students; a lab capable of churning through 20,000 tests a day, with notification of results within hours, not days as is common elsewhere. And the results are equally impressive. The rate of positive test results blipped up to around 3 percent when undergraduates arrived back on campus but that has plummeted to 0.35 percent for the last seven-day period of testing, a tiny fraction of the rate for the nation as a whole. Much of it can be attributed to the closed environment with limited outside contact that might reintroduce the virus.
Still, even while the campus population has dropped by about a third, they are detecting about 250 new infections a week.
The threat of outside contact adding to the risk is why the university amended the undergraduate school calendar to close for Thanksgiving, hold final classes and exams for the semester online, and not return until February.
It doesn't come cheap. Burke estimates it cost $10 million to set up the program and about the same each semester to operate. "But when you compare that to the being able to educate our students, perform research, keep our community thriving, our businesses open, if you add it all up, it's a tremendous return on investment."
Burke acknowledges that they started with some significant advantages. The community is geographically isolated, an electronically linked ID system was already in place for students and employees, they have the ability to control much activity through access to buildings, and they can expel those who do not conform. He believes their system can translate to similar settings but admits, "A big city is very different from a university community." Still, he believes many of those lessons can be translated to different settings.
An alternative story
However, the situation is very different at the University of Colorado, where new infections have surged since undergraduates returned in late August. Administrators recently switched all classes to online only in an attempt to control the virus.
But that wasn't enough for state authorities who cracked down further, just yesterday declaring a two-week lockdown of all students aged 18 to 22, prohibiting gatherings of any size, indoors or out. Students must stay in their rooms except for essential activities, and if any symptoms develop, report for testing. Fraternities and sororities were targeted as past hot spots of infection.
The police will be actively enforcing the lockdown, and violators can face a penalty of up to 90 days in jail and a $1,000 fine.
Skepticism
Public health largely is based upon an appeal to self-interest and altruism, and voluntary compliance with official guidance. Harm reduction often comes into play when an ideal solution meets resistance and coercion plays only a limited role, as when a person with infectious tuberculosis is not compliant with treatment. Many question whether the medical threat of COVID-19 justifies such a sweeping restriction of individual rights of movement and association imposed on everyone simply because of their age and place of residence as is happening in Colorado.
State and federal courts have begun to strike down as an unconstitutional overreach some of the more restrictive decrees to stay at home or close businesses ordered by state and local officials. What was once tolerated as a few weeks or even a few months of restrictions now seems to stretch without an end in sight, and threatens peoples' livelihoods. In this litigious country it seems only a matter of time before someone will challenge some aspects of the Illinois model or similar programs being set up elsewhere as an infringement of their rights.
"I have real concerns about what we have seen over the course of the past several months in terms of going from not enough testing being available to now having more testing [available] because people don't want to be tested, even when they have symptoms," says Michael Osterholm, a noted expert on pandemic preparedness at the University of Minnesota. "We have some college campuses reporting over fifty percent of the students refusing to be tested or refusing to give any of the contacts that might be followed up on."
Often those who have tested positive for the virus "don't want people to know that they're the potential reason there could be an outbreak in their small social circle," says LaQuandra Nesbitt, public health director for Washington, DC. Stigma is one of the main reasons why only 37% of newly infected people have provided names for contact tracing in D.C., and few offer more than a single name.
"We can't test every single person every single day, we would completely go broke, we would be looking at no other health problems. We're not the NFL," says Monica Gandhi. She is a professor of medicine at the University of California San Francisco and works closely with local health officials. "Just because we have a technology doesn't mean that we have to apply it for every purpose that may be indicated. … We would never dream of mass screening the public for influenza."
"Tests don't solve the problem," she argues. Masking is the most crucial piece for Gandhi, along with social distancing, washing hands regularly, and quarantine when testing positive or in contact with someone who is. Those are the actions that break the ongoing spread of transmission. She does support regular testing in high-risk settings such as nursing homes, inpatients in hospitals, and prisons, and periodic surveys in the general population to better understand where the virus is moving.
Drawing from experience with HIV, Gandhi worries that the stigma of a positive result will drive people away from testing. "Low-income persons will be particularly hesitant to get tested, or to share contact information if they do test positive, if they think they may have to quarantine, not work or gain income." That is why San Francisco initially assisted people in isolation with payment of $1285 for two weeks of isolation and other support as part of a right to health program. And this fall, the State of California passed legislation requiring that large businesses continue to pay employees in quarantine.
Tools for self-protection
The American temperament, decentralization, size, administrative complexity, and sheer cost make it highly unlikely that a coercive one-size-fits-all Illinois approach will ever be rolled out from a university campus to the entire nation. People make different decisions in trading off between safety and personal freedom or autonomy, and many are likely to embrace a rapid, inexpensive self-test if one becomes available, much like a home pregnancy test, to proactively monitor their own health.
OraSure Technologies pioneered the first home test for HIV. It is the only over-the-counter saliva test for HIV approved for sale in the U.S. Results show in about 20 minutes. The company went on to develop versions of this test for hepatitis C and Ebola. Thus it came as no surprise when in April the Department of Health and Human Services awarded it a $710 thousand contract to develop a rapid antigen home test for SARS-CoV-2.
Initial optimization studies for the antigen test showed that a nasal sample rather than an oral one generated better results, OraSure president and CEO Stephen Tang told LeapsMag. A test using a nasal swab is expected to be available later this year while work continues to develop an antibody test that uses saliva. He says, "the fundamental challenge is not only to develop the tests but to get it to scale quickly. That's the only way it's really going to matter." The company has manufacturing capacity to produce 35 million tests a year, with about half for SARS-CoV-2, and will double that capacity in steps within the next twelve months, with all of the increased capacity dedicated to COVID-19.
Initial use will be limited to health care workers and by prescription, but the company hopes to make it available over the counter soon after the FDA finalizes its rules on these types of tests for COVID-19. Importantly, OraSure believes its nasal swab test will be able to meet the current FDA standards for at-home tests. No such tests have yet been approved.
Tang says they envision using a phone app with the test, but that's tied to "the question of our century; who owns the data? If you are an individual buying the test, are you really compelled to report to anybody? If you are an employer and you buy the test and your employees take it, are you then entitled to the information because you're the one administering the test? That's all still being debated as well" by regulators, lawyers, and ethicists.
The price hasn't been set but Tang notes that they have "vast experience" in selling directly to the consumer, physicians, and public health systems in the U.S. and in lower-income companies. "We are very aware of what the economics are and what the need is today. We're trying to make this product as widely available to as many people as possible."
Another tool that may help protect the self-motivated are cell phone apps that alert you to potential exposure to others with the virus. Apple, Google and others have developed versions of the app that all work on the same principle and, miraculously, are compatible between the Apple and Android operating system universes. At first glance they look promising.
The glitch is that where they have been available the longest, only about 15-20 percent of users bother to download it, says Bennett Cyphers, a staff technologist with the Electronic Freedom Foundation (EFF), a nonprofit that advocates for privacy and other concerns in cyberspace. He explains, "If 1 in 10 people have the app installed, then only 1 in 100 interactions between everyone is going to be captured by the app. It scales that way; the fewer people you have, then a really, really small fraction of contacts are actually detected."
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives.
Importantly, about 20 percent of Americans do not own a smart phone with the capacity to handle the app; that percentage is even higher among lower income, less educated, older folks who often are most at risk for suffering a severe case of COVID-19. So the value of this tool is likely to remain largely theoretical.
Divining the future
"It's tough to make predictions, especially about the future," the great baseball sage Yogi Berra is reported to have said. Will the COVID-19 pandemic in the U.S. follow the path of Illinois or Colorado?
The recent past often is no guide to such predictions. France, Spain, and Israel once earned plaudits for early and strict enforcement of lockdowns to control spread of the virus and then eased up on those restrictions. At the same time the world watched with condemnation and fascination as Sweden chose to follow a more laissez faire approach, urging voluntary distancing and masking but no major curtailing of activity.
Today the rates of new infections of COVID-19 in the first three countries have exploded to equal or multiples of the rate in Sweden. Which approach was the correct policy? Most people say it is still too early to tell for sure. The same can be said for the examples of Illinois and Colorado.
And then there is the puzzling example of Manaus, the Brazilian city of 1.8 million in the middle of the Amazon which was slammed with infections as hard as New York City; without the medical infrastructure to cope with the virus, 4000 have died. But then, suddenly, new infections began to taper off, and nobody claims to understand why, it certainly wasn't because official policies changed. One guess is that perhaps the region reached herd immunity, but that is simply speculation.
One can pick and choose examples of tough enforcement of quarantine or none to prove their point for the short term. But draconian measures will not be tolerated for long in a free society, and there is no clear, overwhelming evidence that over the long run one policy approach works better than another.
It is important to remember that much of public health is not the result of policy but of what people do in their daily lives. We have come remarkably far in what is still only months since we first heard the name of the virus. Death rates have fallen dramatically as we have learned how to better manage severe disease, often by adapting treatments for other diseases. And there is reason for optimism with the large number of vaccine candidates already in human trials.
We also have learned that we can control much of our own fate through simple but concerted actions in our daily lives such as social distancing, wearing masks, and washing hands. Let's not only remember those facts, but practice them.
Artificial Wombs Are Getting Closer to Reality for Premature Babies
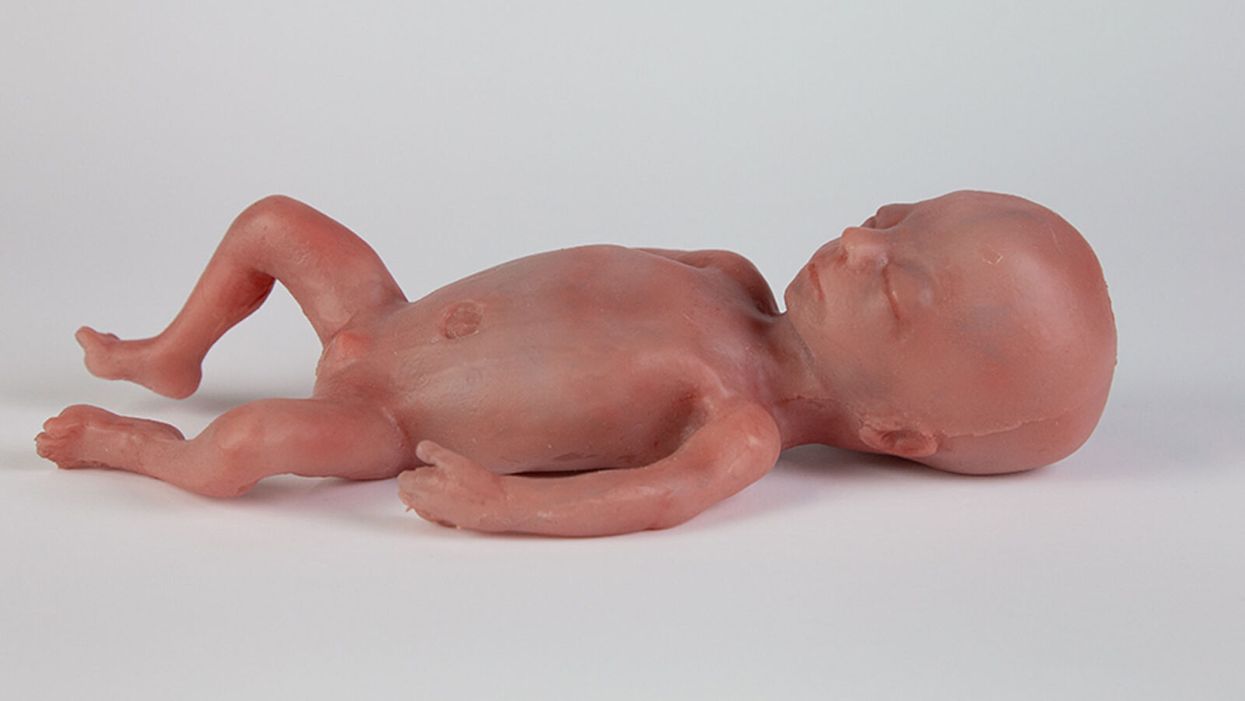
A mannequin of a 24-week-old fetus replicated from MR imaging. Created by: Juliette van Haren, Mark Thielen, Jasper Sterk, Chet Bangaru, and Frank Delbressine, Department of Industrial Design, Eindhoven University of Technology.
In 2017, researchers at the Children's Hospital of Philadelphia grew extremely preterm lambs from hairless to fluffy inside a "biobag," a dark, fluid-filled bag designed to mimic a mother's womb.
"There could be quite a lot of infants that would benefit from artificial womb technologies."
This happened over the course of a month, across a delicate period of fetal development that scientists consider the "edge of viability" for survival at birth.
In 2019, Australian and Japanese scientists repeated the success of keeping extremely premature lambs inside an artificial womb environment until they were ready to survive on their own. Those researchers are now developing a treatment strategy for infants born at "the hard limit of viability," between 20 and 23 weeks of gestation. At the same time, Dutch researchers are going so far as to replicate the sound of a mother's heartbeat inside a biobag. These developments signal exciting times ahead--with a touch of science fiction--for artificial womb technologies. But is there a catch?
"There could be quite a lot of infants that would benefit from artificial womb technologies," says Josephine Johnston, a bioethicist and lawyer at The Hastings Center, an independent bioethics research institute in New York. "These technologies can decrease morbidity and mortality for infants at the edge of viability and help them survive without significant damage to the lungs or other problems," she says.
It is a viewpoint shared by Frans van de Vosse, leader of the Cardiovascular Biomechanics research group at Eindhoven University of Technology in the Netherlands. He participates in a university project that recently received more than $3 million in funding from the E.U. to produce a prototype artificial womb for preterm babies between 24 and 28 weeks of gestation by 2024.
The Eindhoven design comes with a fluid-based environment, just like that of the natural womb, where the baby receives oxygen and nutrients through an artificial placenta that is connected to the baby's umbilical cord. "With current incubators, when a respiratory device delivers oxygen into the lungs in order for the baby to breathe, you may harm preterm babies because their lungs are not yet mature for that," says van de Vosse. "But when the lungs are under water, then they can develop, they can mature, and the baby will receive the oxygen through the umbilical cord, just like in the natural womb," he says.
His research team is working to achieve the "perfectly natural" artificial womb based on strict mathematical models and calculations, van de Vosse says. They are even employing 3D printing technology to develop the wombs and artificial babies to test in them--the mannequins, as van de Vosse calls them. These mannequins are being outfitted with sensors that can replicate the environment a fetus experiences inside a mother's womb, including the soothing sound of her heartbeat.
"The Dutch study's artificial womb design is slightly different from everything else we have seen as it encourages a gestateling to experience the kind of intimacy that a fetus does in pregnancy," says Elizabeth Chloe Romanis, an assistant professor in biolaw at Durham Law School in the U.K. But what is a "gestateling" anyway? It's a term Romanis has coined to describe neither a fetus nor a newborn, but an in-between artificial stage.
"Because they aren't born, they are not neonates," Romanis explains. "But also, they are not inside a pregnant person's body, so they are not fetuses. In an artificial womb the fetus is still gestating, hence why I call it gestateling."
The terminology is not just a semantic exercise to lend a name to what medical dictionaries haven't yet defined. "Gestatelings might have a slightly different psychology," says Romanis. "A fetus inside a mother's womb interacts with the mother. A neonate has some kind of self-sufficiency in terms of physiology. But the gestateling doesn't do either of those things," she says, urging us to be mindful of the still-obscure effects that experiencing early life as a gestateling might have on future humans. Psychology aside, there are also legal repercussions.
The Universal Declaration of Human Rights proclaims the "inalienable rights which everyone is entitled to as a human being," with "everyone" including neonates. However, such a legal umbrella is absent when it comes to fetuses, which have no rights under the same declaration. "We might need a new legal category for a gestateling," concludes Romanis.
But not everyone agrees. "However well-meaning, a new legal category would almost certainly be used to further erode the legality of abortion in countries like the U.S.," says Johnston.
The "abortion war" in the U.S. has risen to a crescendo since 2019, when states like Missouri, Mississippi, Kentucky, Louisiana and Georgia passed so-called "fetal heartbeat bills," which render an abortion illegal once a fetal heartbeat is detected. The situation is only bound to intensify now that Justice Ruth Bader Ginsburg, one of the Supreme Court's fiercest champions for abortion rights, has passed away. If President Trump appoints Ginsburg's replacement, he will probably grant conservatives on the Court the votes needed to revoke or weaken Roe v. Wade, the milestone decision of 1973 that established women's legal right to an abortion.
"A gestateling with intermediate status would almost certainly be considered by some in the U.S. (including some judges) to have at least certain legal rights, likely including right-to-life," says Johnston. This would enable a fetus on the edge of viability to make claims on the mother, and lead either to a shortening of the window in which abortion is legal—or a practice of denying abortion altogether. Instead, Johnston predicts, doctors might offer to transfer the fetus to an artificial womb for external gestation as a new standard of care.
But the legal conundrum does not stop there. The viability threshold is an estimate decided by medical professionals based on the clinical evidence and the technology available. It is anything but static. In the 1970s when Roe v. Wade was decided, for example, a fetus was considered legally viable starting at 28 weeks. Now, with improved technology and medical management, "the hard limit today is probably 20 or 21 weeks," says Matthew Kemp, associate professor at the University of Western Australia and one of the Australian-Japanese artificial womb project's senior researchers.
The changing threshold can result in situations where lots of people invested in the decision disagree. "Those can be hard decisions, but they are case-by-case decisions that families make or parents make with the key providers to determine when to proceed and when to let the infant die. Usually, it's a shared decision where the parents have the final say," says Johnston. But this isn't always the case.
On May 9th 2016, a boy named Alfie Evans was born in Liverpool, UK. Suffering seizures a few months after his birth, Alfie was diagnosed with an unknown neurodegenerative disorder and soon went into a semi-vegetative state, which lasted for more than a year. Alfie's medical team decided to withdraw his ventilation support, suggesting further treatment was unlawful and inhumane, but his parents wanted permission to fly him to a hospital in Rome and attempt to prolong his life there. In the end, the case went all the way up to the Supreme Court, which ruled that doctors could stop providing life support for Alfie, saying that the child required "peace, quiet and privacy." What happened to little Alfie raised huge publicity in the UK and pointedly highlighted the dilemma of whether parents or doctors should have the final say in the fate of a terminally-ill child in life-support treatment.
"In a few years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue."
Alfie was born and, thus had legal rights, yet legal and ethical mayhem arose out of his case. When it comes to gestatelings, the scenarios will be even more complicated, says Romanis. "I think there's a really big question about who has parental rights and who doesn't," she says. "The assisted reproductive technology (ART) law in the U.K. hasn't been updated since 2008....It certainly needs an update when you think about all the things we have done since [then]."
This June, for instance, scientists from the Wake Forest Institute for Regenerative Medicine in North Carolina published research showing that they could take a small sample of tissue from a rabbit's uterus and create a bioengineered uterus, which then supported both fertilization and normal pregnancy like a natural uterus does.
"In [a number of] years from now, women who cannot get pregnant because of uterine infertility will be able to have a fully functional uterus made from their own tissue," says Dr. Anthony Atala, the Institute's director and a pioneer in regenerative medicine. These bioengineered uteri will eventually be covered by insurance, Atala expects. But when it comes to artificial wombs that externally gestate premature infants, will all mothers have equal access?
Medical reports have already shown racial and ethnic disparities in infertility treatments and access to assisted reproductive technologies. Costs on average total $12,400 per cycle of treatment and may require several cycles to achieve a live birth. "There's no indication that artificial wombs would be treated any differently. That's what we see with almost every expensive new medical technology," says Johnston. In a much more dystopian future, there is even a possibility that inequity in healthcare might create disturbing chasms in how women of various class levels bear children. Romanis asks us to picture the following scenario:
We live in a world where artificial wombs have become mainstream. Most women choose to end their pregnancies early and transfer their gestatelings to the care of machines. After a while, insurers deem full-term pregnancy and childbirth a risky non-necessity, and are lobbying to stop covering them altogether. Wealthy white women continue opting out of their third trimesters (at a high cost), since natural pregnancy has become a substandard route for poorer women. Those women are strongly judged for any behaviors that could risk their fetus's health, in contrast with the machine's controlled environment. "Why are you having a coffee during your pregnancy?" critics might ask. "Why are you having a glass of red wine? If you can't be perfect, why don't you have it the artificial way?"
Problem is, even if they want to, they won't be able to afford it.
In a more sanguine version, however, the artificial wombs are only used in cases of prematurity as a life-saving medical intervention rather than as a lifestyle accommodation. The 15 million babies who are born prematurely each year and may face serious respiratory, cardiovascular, visual and hearing problems, as well as learning disabilities, instead continue their normal development in artificial wombs. After lots of deliberation, insurers agree to bear the cost of external wombs because they are cheaper than a lifetime of medical care for a disabled or diseased person. This enables racial and ethnic minority women, who make up the majority of women giving premature birth, to access the technology.
Even extremely premature babies, those babies (far) below the threshold of 28 weeks of gestation, half of which die, could now discover this thing called life. In this scenario, as the Australian researcher Kemp says, we are simply giving a good shot at healthy, long-term survival to those who were unfortunate enough to start too soon.