Deep Brain Stimulation for Mental Illnesses Raises Ethical Concerns
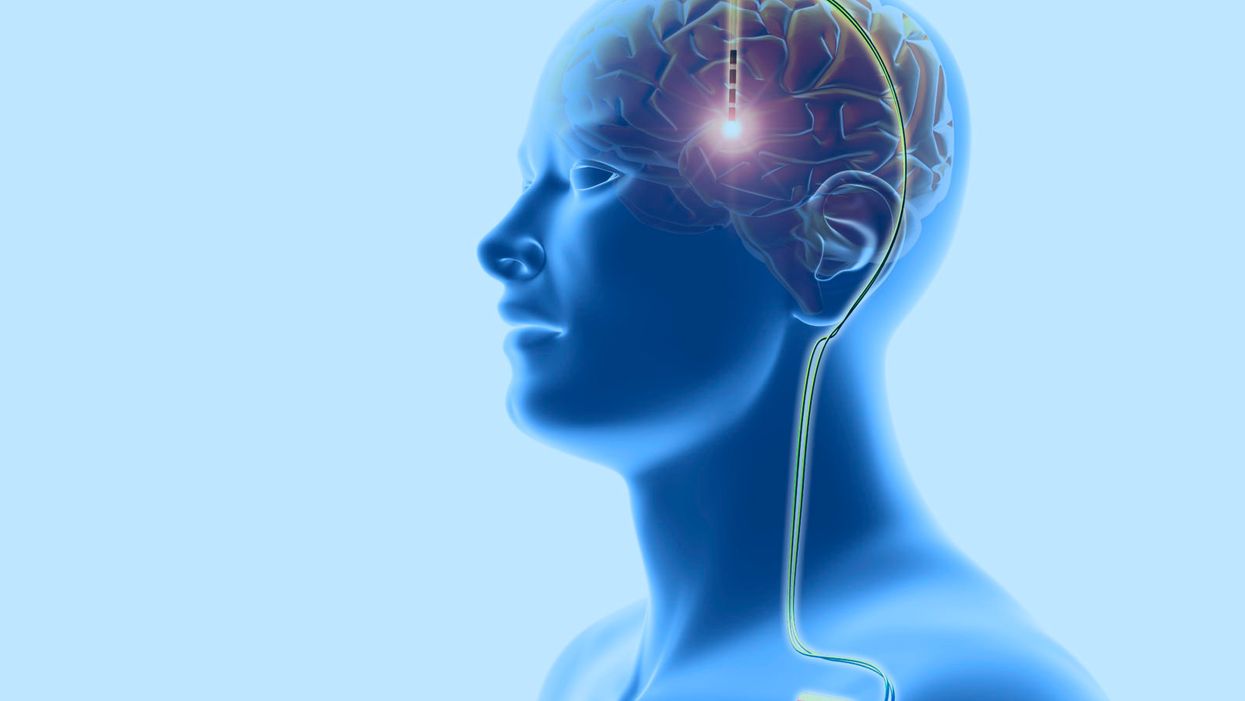
Deep brain stimulation: This neurosurgical treatment involves the implantation of electrodes in the cerebral lobes of the brain, linked through the scalp (top) to wires (down right) leading to a battery implanted below the skin. This sends electrical impulses to specific areas of the brain. DBS was developed for the treatment of Parkinson's disease, but is being investigated for use in other conditions.
Imagine that you are one of the hundreds of millions of people who suffer from depression. Medication hasn't helped you, so you're looking for another treatment option. Something powerful enough to change your mood as soon as you need a lift.
"If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature?"
Enter deep brain stimulation: a type of therapy in which one or more electrodes are inserted into your brain and connected to a surgically implanted, battery-operated medical device in your chest. This device, which is approximately the size of a stopwatch, sends electric pulses to a targeted region of your brain. The idea is to control a variety of neurological symptoms that can't be adequately managed by drugs.
Over the last twenty years, deep brain stimulation, known as DBS, has become an efficient and safe alternative for the treatment of chronic neurological diseases such as epilepsy, Parkinson's disease and neuropathic pain. According to the International Neuromodulation Society, there have been more than 80,000 deep brain stimulation implants performed around the world.
The Food and Drug Administration approved DBS as a treatment for essential tremor and Parkinson's in 1997, dystonia in 2003 and obsessive compulsive disorder in 2009. Since doctors can use drugs and treatments "off-label" (not approved by the FDA) to treat patients with any disease, DBS is now also being investigated as a treatment for chronic pain, PTSD and major depression.
And these new applications are raising profound ethical questions about individuality, personality, and even what it means to be human.
"These patients are essentially having a computer that can modify and influence emotional processing, mood and motor outputs inserted into the brain," said Gabriel Lazaro-Munoz, an assistant professor at The Center for Medical Ethics and Health Policy at Baylor College of Medicine. "These responses define us as human beings and dictate our autonomy. If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature? These are some of the questions we have to consider."
"When we are not in control of ourselves, are we ourselves?"
The U.S. government has similar concerns about DBS. The National Institutes of Health recently awarded grants to study the neuroethical issues surrounding the use of DBS in neuropsychiatric and movement disorders and appropriate consent for brain research. The grants are part of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. Walter Koroshetz, director of NIH's National Institute of Neurological Disorders and Stroke said, "Neuroscience is rapidly moving toward a new frontier of research on human brains that may have long-lasting and unforeseen effects. These new awards signal our commitment to research conducted in a responsible way as to anticipate all potential consequences, and to ensure that research subjects have a clear understanding of the potential benefits and risks of participating in studies."
Dr. Lazaro-Munoz's Center was awarded one of the grants to identify and evaluate the ethical, legal and social concerns with adaptive deep brain stimulation (aDBS) technologies. Adaptive DBS is a relatively new version of the technology that enables recording of brain cell activity that is then used to regulate the brain in real time. He and his team will closely observe researchers conducting aDBS studies and administering in-depth interviews to trial participants, their caregivers, and researchers, as well as individuals who declined to participate in such studies. The goal is to gain a better understanding of the ethical concerns at stake in order to guide responsible research.
Dr. Lazaro-Munoz said one of the concerns is dehumanization. "By using this technology are we compromising what makes us human? When we are not in control of ourselves, are we ourselves?" He notes that similar concerns were raised about pharmaceutical treatments for illnesses. "Both change behaviors and emotional processing. However, there is a difference. Culturally we are more used to using drugs, not implanting devices into brain and computer interfaces. Many people think of it as science fiction."
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish.
Pills for OCD and depression take longer than DBS to see significant improvement, sometimes months. "A DBS device is either on or off. And patients and families see changes immediately," Dr. Lazaro-Munoz said. "Family members are often startled by these changes, as are the patients." He's observed that patients feel more in control with pills because they can alter and "play" with the dose or even skip a dose.
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish, like in this must-see video.
But surgical procedures to treat motor symptoms are also increasingly being implicated as a cause of behavioral changes, both positive and negative, in patients with Parkinson's. The personality changes reported in patients who undergo DBS include hypermania, pathological gambling, hypersexuality, impulsivity and aggressiveness. One patient who suffered from OCD fell in love with the music of Johnny Cash when his brain was stimulated. On the positive side, patients report memory enhancement.
One patient who is pleased with DBS is Greg Barstead, who was diagnosed with Parkinson's in 2003, when he was the president of Colonial Penn Life Insurance Company. He also has dystonia, which affects his neck and shoulders. Barstead said that DBS has been helpful for a range of symptoms: "My shoulder is a lot less stiff and my neck hurts less. And my tremors are under control. It is not perfect, as it doesn't relieve all the Parkinson's symptoms, but it does enough of a good job that both my wife and I are very happy I had DBS."
"We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device."
He said he hasn't noticed any personality changes, but noted that the disease itself can cause such changes. In fact, studies have shown that it can cause many psychiatric problems including depression and hallucinations. And, approximately a third of Parkinson's patients develop dementia.
Arthur L. Caplan, founding head of the Division of Medical Ethics at NYU School of Medicine, notes that unlike psychosurgery, DBS can be turned on and off and the device can be removed. "There are less ethical concerns around treating patients with Parkinson's disease than other illnesses because surgeons know exactly where to implant the device and have many years of experience with it," he said, adding that he is concerned about using DBS for other illnesses, such as depression. "We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device. And I would certainly not advocate its use in patients with mild depression."
Dr. Lazaro-Munoz said of the personality changes possible with DBS, physicians need to consider how the patients were functioning without it. "Patients who are candidates for DBS typically used many medications as well as psychotherapy before opting for DBS," he explained. "To me, the question is what is the net result of using this technology? Does the patient have regrets? Are the changes in personality significant or not? Although most DBS patients report being happy they underwent the procedure, some say they don't feel like themselves after DBS. Others feel they are more like themselves, especially if there are dramatic improvements in movement problems or relief of OCD symptoms."
And then there is the question of money. The costs of DBS are covered by most insurance companies and Medicare only for FDA-approved targets like Parkinson's. Off-label uses are not covered, at least for now.
Caplan reminds people that DBS devices are manufactured by companies that are interested in making money and the average cost per treatment is around $50,000. "I am interested in seeing DBS move forward," he said. "But we must be careful and not allow industry to make it go too fast, or be used on too many people, before we know it is effective."
New implants let paraplegics surf the web and play computer games
Rodney Gorham, an Australian living with ALS, has reconnected with the world, thanks to a brain-machine interface called the Stentrode.
When I greeted Rodney Gorham, age 63, in an online chat session, he replied within seconds: “My pleasure.”
“Are you moving parts of your body as you type?” I asked.
This time, his response came about five minutes later: “I position the cursor with the eye tracking and select the same with moving my ankles.” Gorham, a former sales representative from Melbourne, Australia, living with amyotrophic lateral sclerosis, or ALS, a rare form of Lou Gehrig’s disease that impairs the brain’s nerve cells and the spinal cord, limiting the ability to move. ALS essentially “locks” a person inside their own body. Gorham is conversing with me by typing with his mind only–no fingers in between his brain and his computer.
The brain-computer interface enabling this feat is called the Stentrode. It's the brainchild of Synchron, a company backed by Amazon’s Jeff Bezos and Microsoft cofounder Bill Gates. After Gorham’s neurologist recommended that he try it, he became one of the first volunteers to have an 8mm stent, laced with small electrodes, implanted into his jugular vein and guided by a surgeon into a blood vessel near the part of his brain that controls movement.
After arriving at their destination, these tiny sensors can detect neural activity. They relay these messages through a small receiver implanted under the skin to a computer, which then translates the information into words. This minimally invasive surgery takes a day and is painless, according to Gorham. Recovery time is typically short, about two days.
When a paralyzed patient thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts.
When a paralyzed patient such as Gorham thinks about trying to move their arms or legs, the motor cortex will fire patterns that are specific to the patient’s thoughts. This pattern is detected by the Stentrode and relayed to a computer that learns to associate this pattern with the patient’s physical movements. The computer recognizes thoughts about kicking, making a fist and other movements as signals for clicking a mouse or pushing certain letters on a keyboard. An additional eye-tracking device controls the movement of the computer cursor.
The process works on a letter by letter basis. That’s why longer and more nuanced responses often involve some trial and error. “I have been using this for about two years, and I enjoy the sessions,” Gorham typed during our chat session. Zafar Faraz, field clinical engineer at Synchron, sat next to Gorham, providing help when required. Gorham had suffered without internet access, but now he looks forward to surfing the web and playing video games.

Gorham, age 63, has been enjoying Stentrode sessions for about two years.
Rodeny Dekker
The BCI revolution
In the summer of 2021, Synchron became the first company to receive the FDA’s Investigational Device Exemption, which allows research trials on the Stentrode in human patients. This past summer, the company, together with scientists from Icahn School of Medicine at Mount Sinai and the Neurology and Neurosurgery Department at Utrecht University, published a paper offering a framework for how to develop BCIs for patients with severe paralysis – those who can't use their upper limbs to type or use digital devices.
Three months ago, Synchron announced the enrollment of six patients in a study called COMMAND based in the U.S. The company will seek approval next year from the FDA to make the Stentrode available for sale commercially. Meanwhile, other companies are making progress in the field of BCIs. In August, Neuralink announced a $280 million financing round, the biggest fundraiser yet in the field. Last December, Synchron announced a $75 million financing round. “One thing I can promise you, in five years from now, we’re not going to be where we are today. We're going to be in a very different place,” says Elad I. Levy, professor of neurosurgery and radiology at State University of New York in Buffalo.
The risk of hacking exists, always. Cybercriminals, for example, might steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices while extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“The prospect of bestowing individuals with paralysis a renewed avenue for communication and motor functionality is a step forward in neurotech,” says Hayley Nelson, a neuroscientist and founder of The Academy of Cognitive and Behavioral Neuroscience. “It is an exciting breakthrough in a world of devastating, scary diseases,” says Neil McArthur, a professor of philosophy and director of the Centre for Professional and Applied Ethics at the University of Manitoba. “To connect with the world when you are trapped inside your body is incredible.”
While the benefits for the paraplegic community are promising, the Stentrode’s long-term effectiveness and overall impact needs more research on safety. “Potential risks like inflammation, damage to neural tissue, or unexpected shifts in synaptic transmission due to the implant warrant thorough exploration,” Nelson says.
There are also concens about data privacy concerns and the policies of companies to safeguard information processed through BCIs. “Often, Big Tech is ahead of the regulators because the latter didn’t envisage such a turn of events...and companies take advantage of the lack of legal framework to push forward,” McArthur says. Hacking is another risk. Cybercriminals could steal sensitive personal data for financial reasons, blackmailing, or to spread malware to other connected devices. Extremist groups could potentially hack BCIs to manipulate individuals into supporting their causes or carrying out actions on their behalf.
“We have to protect patient identity, patient safety and patient integrity,” Levy says. “In the same way that we protect our phones or computers from hackers, we have to stay ahead with anti-hacking software.” Even so, Levy thinks the anticipated benefits for the quadriplegic community outweigh the potential risks. “We are on the precipice of an amazing technology. In the future, we would be able to connect patients to peripheral devices that enhance their quality of life.”
In the near future, the Stentrode could enable patients to use the Stentrode to activate their wheelchairs, iPods or voice modulators. Synchron's focus is on using its BCI to help patients with significant mobility restrictions—not to enhance the lives of healthy people without any illnesses. Levy says we are not prepared for the implications of endowing people with superpowers.
I wondered what Gorham thought about that. “Pardon my question, but do you feel like you have sort of transcended human nature, being the first in a big line of cybernetic people doing marvelous things with their mind only?” was my last question to Gorham.
A slight smile formed on his lips. In less than a minute, he typed: “I do a little.”
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation

