Deep Brain Stimulation for Mental Illnesses Raises Ethical Concerns
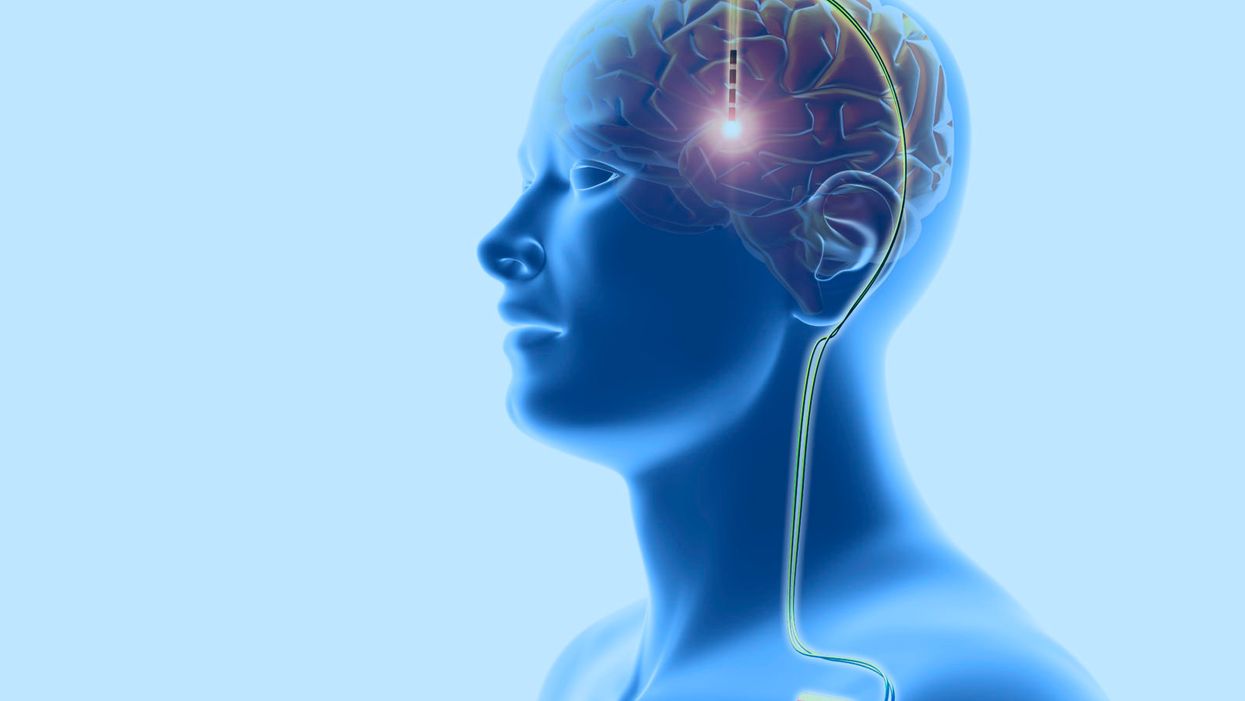
Deep brain stimulation: This neurosurgical treatment involves the implantation of electrodes in the cerebral lobes of the brain, linked through the scalp (top) to wires (down right) leading to a battery implanted below the skin. This sends electrical impulses to specific areas of the brain. DBS was developed for the treatment of Parkinson's disease, but is being investigated for use in other conditions.
Imagine that you are one of the hundreds of millions of people who suffer from depression. Medication hasn't helped you, so you're looking for another treatment option. Something powerful enough to change your mood as soon as you need a lift.
"If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature?"
Enter deep brain stimulation: a type of therapy in which one or more electrodes are inserted into your brain and connected to a surgically implanted, battery-operated medical device in your chest. This device, which is approximately the size of a stopwatch, sends electric pulses to a targeted region of your brain. The idea is to control a variety of neurological symptoms that can't be adequately managed by drugs.
Over the last twenty years, deep brain stimulation, known as DBS, has become an efficient and safe alternative for the treatment of chronic neurological diseases such as epilepsy, Parkinson's disease and neuropathic pain. According to the International Neuromodulation Society, there have been more than 80,000 deep brain stimulation implants performed around the world.
The Food and Drug Administration approved DBS as a treatment for essential tremor and Parkinson's in 1997, dystonia in 2003 and obsessive compulsive disorder in 2009. Since doctors can use drugs and treatments "off-label" (not approved by the FDA) to treat patients with any disease, DBS is now also being investigated as a treatment for chronic pain, PTSD and major depression.
And these new applications are raising profound ethical questions about individuality, personality, and even what it means to be human.
"These patients are essentially having a computer that can modify and influence emotional processing, mood and motor outputs inserted into the brain," said Gabriel Lazaro-Munoz, an assistant professor at The Center for Medical Ethics and Health Policy at Baylor College of Medicine. "These responses define us as human beings and dictate our autonomy. If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature? These are some of the questions we have to consider."
"When we are not in control of ourselves, are we ourselves?"
The U.S. government has similar concerns about DBS. The National Institutes of Health recently awarded grants to study the neuroethical issues surrounding the use of DBS in neuropsychiatric and movement disorders and appropriate consent for brain research. The grants are part of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. Walter Koroshetz, director of NIH's National Institute of Neurological Disorders and Stroke said, "Neuroscience is rapidly moving toward a new frontier of research on human brains that may have long-lasting and unforeseen effects. These new awards signal our commitment to research conducted in a responsible way as to anticipate all potential consequences, and to ensure that research subjects have a clear understanding of the potential benefits and risks of participating in studies."
Dr. Lazaro-Munoz's Center was awarded one of the grants to identify and evaluate the ethical, legal and social concerns with adaptive deep brain stimulation (aDBS) technologies. Adaptive DBS is a relatively new version of the technology that enables recording of brain cell activity that is then used to regulate the brain in real time. He and his team will closely observe researchers conducting aDBS studies and administering in-depth interviews to trial participants, their caregivers, and researchers, as well as individuals who declined to participate in such studies. The goal is to gain a better understanding of the ethical concerns at stake in order to guide responsible research.
Dr. Lazaro-Munoz said one of the concerns is dehumanization. "By using this technology are we compromising what makes us human? When we are not in control of ourselves, are we ourselves?" He notes that similar concerns were raised about pharmaceutical treatments for illnesses. "Both change behaviors and emotional processing. However, there is a difference. Culturally we are more used to using drugs, not implanting devices into brain and computer interfaces. Many people think of it as science fiction."
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish.
Pills for OCD and depression take longer than DBS to see significant improvement, sometimes months. "A DBS device is either on or off. And patients and families see changes immediately," Dr. Lazaro-Munoz said. "Family members are often startled by these changes, as are the patients." He's observed that patients feel more in control with pills because they can alter and "play" with the dose or even skip a dose.
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish, like in this must-see video.
But surgical procedures to treat motor symptoms are also increasingly being implicated as a cause of behavioral changes, both positive and negative, in patients with Parkinson's. The personality changes reported in patients who undergo DBS include hypermania, pathological gambling, hypersexuality, impulsivity and aggressiveness. One patient who suffered from OCD fell in love with the music of Johnny Cash when his brain was stimulated. On the positive side, patients report memory enhancement.
One patient who is pleased with DBS is Greg Barstead, who was diagnosed with Parkinson's in 2003, when he was the president of Colonial Penn Life Insurance Company. He also has dystonia, which affects his neck and shoulders. Barstead said that DBS has been helpful for a range of symptoms: "My shoulder is a lot less stiff and my neck hurts less. And my tremors are under control. It is not perfect, as it doesn't relieve all the Parkinson's symptoms, but it does enough of a good job that both my wife and I are very happy I had DBS."
"We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device."
He said he hasn't noticed any personality changes, but noted that the disease itself can cause such changes. In fact, studies have shown that it can cause many psychiatric problems including depression and hallucinations. And, approximately a third of Parkinson's patients develop dementia.
Arthur L. Caplan, founding head of the Division of Medical Ethics at NYU School of Medicine, notes that unlike psychosurgery, DBS can be turned on and off and the device can be removed. "There are less ethical concerns around treating patients with Parkinson's disease than other illnesses because surgeons know exactly where to implant the device and have many years of experience with it," he said, adding that he is concerned about using DBS for other illnesses, such as depression. "We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device. And I would certainly not advocate its use in patients with mild depression."
Dr. Lazaro-Munoz said of the personality changes possible with DBS, physicians need to consider how the patients were functioning without it. "Patients who are candidates for DBS typically used many medications as well as psychotherapy before opting for DBS," he explained. "To me, the question is what is the net result of using this technology? Does the patient have regrets? Are the changes in personality significant or not? Although most DBS patients report being happy they underwent the procedure, some say they don't feel like themselves after DBS. Others feel they are more like themselves, especially if there are dramatic improvements in movement problems or relief of OCD symptoms."
And then there is the question of money. The costs of DBS are covered by most insurance companies and Medicare only for FDA-approved targets like Parkinson's. Off-label uses are not covered, at least for now.
Caplan reminds people that DBS devices are manufactured by companies that are interested in making money and the average cost per treatment is around $50,000. "I am interested in seeing DBS move forward," he said. "But we must be careful and not allow industry to make it go too fast, or be used on too many people, before we know it is effective."
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

