A Tool for Disease Detection Is Right Under Our Noses
In March, researchers published a review that lists which organic chemicals match up with certain diseases and biomarkers in the skin, saliva and urine. It’s an important step in creating a robot nose that can reliably detect diseases.
The doctor will sniff you now? Well, not on his or her own, but with a device that functions like a superhuman nose. You’ll exhale into a breathalyzer, or a sensor will collect “scent data” from a quick pass over your urine or blood sample. Then, AI software combs through an olfactory database to find patterns in the volatile organic compounds (VOCs) you secreted that match those associated with thousands of VOC disease biomarkers that have been identified and cataloged.
No further biopsy, imaging test or procedures necessary for the diagnosis. According to some scientists, this is how diseases will be detected in the coming years.
All diseases alter the organic compounds found in the body and their odors. Volatolomics is an emerging branch of chemistry that uses the smell of gases emitted by breath, urine, blood, stool, tears or sweat to diagnose disease. When someone is sick, the normal biochemical process is disrupted, and this alters the makeup of the gas, including a change in odor.
“These metabolites show a snapshot of what’s going on with the body,” says Cristina Davis, a biomedical engineer and associate vice chancellor of Interdisciplinary Research and Strategic Initiatives at the University of California, Davis. This opens the door to diagnosing conditions even before symptoms are present. It’s possible to detect a sweet, fruity smell in the breath of someone with diabetes, for example.
Hippocrates may have been the first to note that people with certain diseases give off an odor but dogs provided the proof of concept. Scientists have published countless studies in which dogs or other high-performing smellers like rodents have identified people with cancer, lung disease or other conditions by smell alone. The brain region that analyzes smells is proportionally about 40 times greater in dogs than in people. The noses of rodents are even more powerful.
Take prostate cancer, which is notoriously difficult to detect accurately with standard medical testing. After sniffing a tiny urine sample, trained dogs were able to pick out prostate cancer in study subjects more than 96 percent of the time, and earlier than a physician could in some cases.
But using dogs as bio-detectors is not practical. It is labor-intensive, complicated and expensive to train dogs to bark or lie down when they smell a certain VOC, explains Bruce Kimball, a chemical ecologist at the Monell Chemical Senses Center in Philadelphia. Kimball has trained ferrets to scratch a box when they smell a specific VOC so he knows. The lab animal must be taught to distinguish the VOC from background odors and trained anew for each disease scent.

In the lab of chemical ecologist Bruce Kimball, ferrets were trained to scratch a box when they identified avian flu in mallard ducks.
Glen J. Golden
There are some human super-smellers among us. In 2019, Joy Milne of Scotland proved she could unerringly identify people with Parkinson’s disease from a musky scent emitted from their skin. Clinical testing showed that she could distinguish the odor of Parkinson’s on a worn t-shirt before clinical symptoms even appeared.
Hossam Haick, a professor at Technion-Israel Institute of Technology, maintains that volatolomics is the future of medicine. Misdiagnosis and late detection are huge problems in health care, he says. “A precise and early diagnosis is the starting point of all clinical activities.” Further, this science has the potential to eliminate costly invasive testing or imaging studies and improve outcomes through earlier treatment.
The Nose Knows a Lot
“Volatolomics is not a fringe theory. There is science behind it,” Davis stresses. Every VOC has its own fingerprint, and a method called gas chromatography-mass spectrometry (GCMS) uses highly sensitive instruments to separate the molecules of these VOCs to determine their structures. But GCMS can’t discern the telltale patterns of particular diseases, and other technologies to analyze biomarkers have been limited.
We have technology that can see, hear and sense touch but scientists don’t have a handle yet on how smell works. The ability goes beyond picking out a single scent in someone’s breath or blood sample. It’s the totality of the smell—not the smell of a single chemical— which defines a disease. The dog’s brain is able to infer something when they smell a VOC that eludes human analysis so far.
Odor is a complex ecosystem and analyzing a VOC is compounded by other scents in the environment, says Kimball. A person’s diet and use of tobacco or alcohol also will affect the breath. Even fluctuations in humidity and temperature can contaminate a sample.
If successful, a sophisticated AI network can imitate how the dog brain recognizes patterns in smells. Early versions of robot noses have already been developed.
With today’s advances in data mining, AI and machine learning, scientists are trying to create mechanical devices that can draw on algorithms based on GCMS readings and data about diseases that dogs have sniffed out. If successful, a sophisticated AI network can imitate how the dog brain recognizes patterns in smells.
In March, Nano Research published a comprehensive review of volatolomics in health care authored by Haick and seven colleagues. The intent was to bridge gaps in the field for scientists trying to connect the biomarkers and sensor technology needed to develop a robot nose. This paper serves as a reference manual for the field that lists which VOCs are associated with what disease and the biomarkers in skin, saliva, breath, and urine.
Weiwei Wu, one of the co-authors and a professor at Xidian University in China, explains that creating a robotic nose requires the expertise of chemists, computer scientists, electrical engineers, material scientists, and clinicians. These researchers use different terms and methodologies and most have not collaborated before with the other disciplines. “The electrical engineers know the device but they don’t know as much about the biomarkers they need to detect,” Wu offers as an example.
This review is significant, Wu continues, because it can facilitate progress in the field by providing experts in all the disciplines with the basic knowledge needed to create an effective robot nose for diagnostic use. The paper also includes a systematic summary of the research methodology of volatolomics.
Once scientists build a stronger database of VOCs, they can program a device to identify critical patterns of specified diseases on a reliable basis. On a machine learning model, the algorithms automatically get better at diagnosing with each use. Wu envisions further tweaks in the next few years to make the devices smaller and consume less power.
A Whiff of the Future
Early versions of robot noses have already been developed. Some of them use chemical sensors to pick up smells in the breath or other body emission molecules. That data is sent through an electrical signal to a computer network for interpretation and possible linkage to a disease.
This electronic nose, or e-nose, has been successful in small pilot studies at labs around the world. At Ben-Gurion University in Israel, researchers detected breast cancer with electronic gas sensors with 95% accuracy, a higher sensitivity than mammograms. Other robot noses, called p-noses, use photons instead of electrical signals.
The mechanical noses being developed tap different methodologies and analytic techniques which makes it hard to compare them. Plus, the devices are intended for varying uses. One team, for example, is working on an e-nose that can be waved over a plate to screen for the presence of a particular allergen when you’re dining out.
A robot nose could be used as a real-time diagnostic tool in clinical practice. Kimball is working on one such tool that can distinguish between a viral and bacterial infection. This would enable physicians to determine whether an antibiotic prescription is appropriate without waiting for a lab result.
Davis is refining a hand-held device that identifies COVID-19 through a simple breath test. She sees the tool being used at crowded airports, sports stadiums and concert venues where PCR or rapid antigen testing is impractical. Background air samples are collected from the space so that those signals can be removed from the human breath measurement. “[The sensor tool] has the same accuracy as the rapid antigen test kits but exhaled breath is easier to collect,” she notes.

The NaNose, also known as the SniffPhone, uses tiny sensors boosted by AI to distinguish Alzheimer's, Crohn's disease, the early stages of several cancers, and other diseases with 84 to 98 percent accuracy.
Hossam Haick
Haick named his team’s robot nose, “NaNose,” since it is based on nanotechnology; the prototype is called the SniffPhone. Using tiny sensors boosted by AI, it can distinguish 23 diseases in human subjects with 84 to 98 percent accuracy. This includes early stages of several cancers, Alzheimer’s, tuberculosis and Crohn’s disease. His team has been raising the accuracy level by combining biomarker signals from both breath and skin, for example. The goal is to achieve 99.9 percent accuracy consistently so no other diagnostic tests would be needed before treating the patient. Plus, it will be affordable, he says.
Kimball predicts we’ll be seeing these diagnostic tools in the next decade. “The physician would narrow down what [the diagnosis] might be and then get the correct tool,” he says. Others are envisioning one device that can screen for multiple diseases by programming the software, which would be updated regularly with new findings.
Larger volatolomics studies must be conducted before these e-noses are ready for clinical use, however. Experts also need to learn how to establish normal reference ranges for e-nose readings to support clinicians using the tool.
“Taking successful prototypes from the lab to industry is the challenge,” says Haick, ticking off issues like reproducibility, mass production and regulation. But volatolomics researchers are unanimous in believing the future of health care is so close they can smell it.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
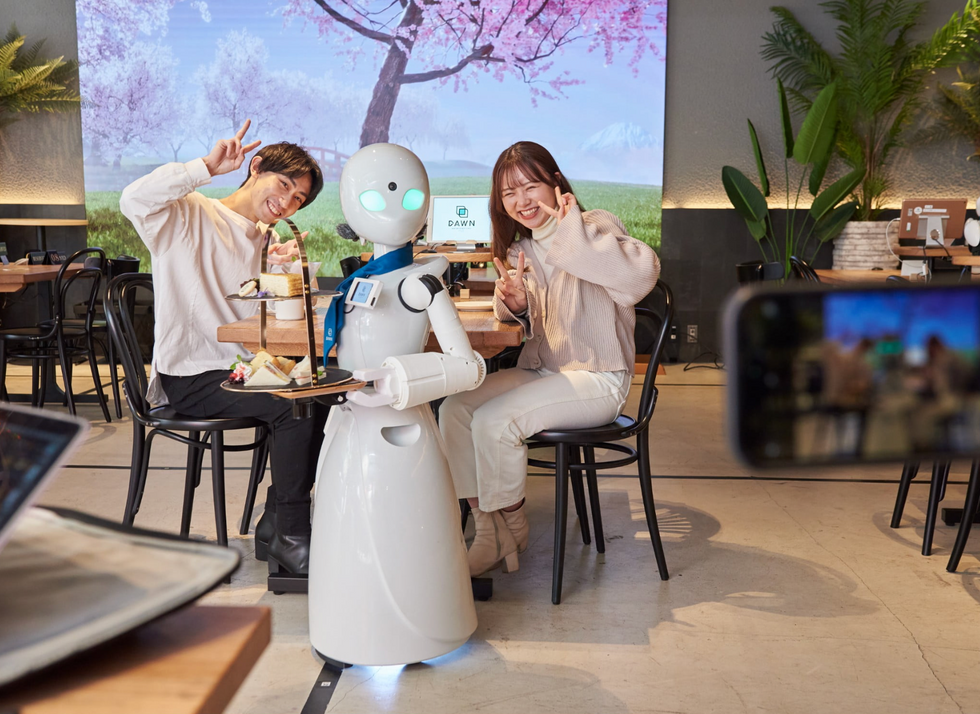
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
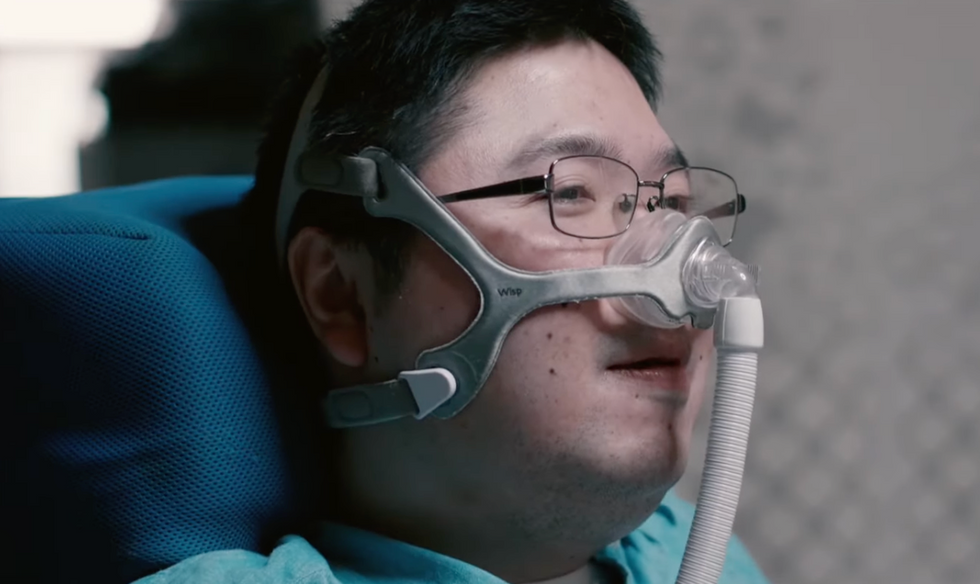
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

