How dozens of men across Alaska (and their dogs) teamed up to save one town from a deadly outbreak

In 1925, health officials in Alaska came up with a creative solution to save a remote fishing town from a deadly disease outbreak.
During the winter of 1924, Curtis Welch – the only doctor in Nome, a remote fishing town in northwest Alaska – started noticing something strange. More and more, the children of Nome were coming to his office with sore throats.
Initially, Welch dismissed the cases as tonsillitis or some run-of-the-mill virus – but when more kids started getting sick, with some even dying, he grew alarmed. It wasn’t until early 1925, after a three-year-old boy died just two weeks after becoming ill, that Welch realized that his worst suspicions were true. The boy – and dozens of other children in town – were infected with diphtheria.
A DEADLY BACTERIA
Diphtheria is nearly nonexistent and almost unheard of in industrialized countries today. But less than a century ago, diphtheria was a household name – one that struck fear in the heart of every parent, as it was extremely contagious and particularly deadly for children.
Diphtheria – a bacterial infection – is an ugly disease. When it strikes, the bacteria eats away at the healthy tissues in a patient’s respiratory tract, leaving behind a thick, gray membrane of dead tissue that covers the patient's nose, throat, and tonsils. Not only does this membrane make it very difficult for the patient to breathe and swallow, but as the bacteria spreads through the bloodstream, it causes serious harm to the heart and kidneys. It sometimes also results in nerve damage and paralysis. Even with treatment, diphtheria kills around 10 percent of people it infects. Young children, as well as adults over the age of 60, are especially at risk.
Welch didn’t suspect diphtheria at first. He knew the illness was incredibly contagious and reasoned that many more people would be sick – specifically, the family members of the children who had died – if there truly was an outbreak. Nevertheless, the symptoms, along with the growing number of deaths, were unmistakable. By 1925 Welch knew for certain that diphtheria had come to Nome.
In desperation, Welch tried treating an infected seven-year-old girl with some expired antitoxin – but she died just a few hours after he administered it.
AN INACCESSIBLE CURE
A vaccine for diphtheria wouldn’t be widely available until the mid-1930s and early 1940s – so an outbreak of the disease meant that each of the 10,000 inhabitants of Nome were all at serious risk.
One option was to use something called an antitoxin – a serum consisting of anti-diphtheria antibodies – to treat the patients. However, the town’s reserve of diphtheria antitoxin had expired. Welch had ordered a replacement shipment of antitoxin the previous summer – but the shipping port that was set to deliver the serum had been closed due to ice, and no new antitoxin would arrive before spring of 1925. In desperation, Welch tried treating an infected seven-year-old girl with some expired antitoxin – but she died just a few hours after he administered it.
Welch radioed for help to all the major towns in Alaska as well as the US Public Health Service in Washington, DC. His telegram read: An outbreak of diphtheria is almost inevitable here. I am in urgent need of one million units of diphtheria antitoxin. Mail is the only form of transportation.
FOUR-LEGGED HEROES
When the Alaskan Board of Health learned about the outbreak, the men rushed to devise a plan to get antitoxin to Nome. Dropping the serum in by airplane was impossible, as the available planes were unsuitable for flying during Alaska’s severe winter weather, where temperatures were routinely as cold as -50 degrees Fahrenheit.
In late January 1925, roughly 30,000 units of antitoxin were located in an Anchorage hospital and immediately delivered by train to a nearby city, Nenana, en route to Nome. Nenana was the furthest city that was reachable by rail – but unfortunately it was still more than 600 miles outside of Nome, with no transportation to make the delivery. Meanwhile, Welch had confirmed 20 total cases of diphtheria, with dozens more at high risk. Diphtheria was known for wiping out entire communities, and the entire town of Nome was in danger of suffering the same fate.
It was Mark Summer, the Board of Health superintendent, who suggested something unorthodox: Using a relay team of sled-racing dogs to deliver the antitoxin serum from Nenana to Nome. The Board quickly voted to accept Summer’s idea and set up a plan: The thousands of units of antitoxin serum would be passed along from team to team at different towns along the mail route from Nenana to Nome. When it reached a town called Nulato, a famed dogsled racer named Leonhard Seppala and his experienced team of huskies would take the serum more than 90 miles over the ice of Norton Sound, the longest and most treacherous part of the journey. Past the sound, the serum would change hands several times more before arriving in Nome.
Between January 27 and 31, the serum passed through roughly a dozen drivers and their dog sled teams, each of them carrying the serum between 20 and 50 miles to the next destination. Though each leg of the trip took less than a day, the sub-zero temperatures – sometimes as low as -85 degrees – meant that every driver and dog risked their lives. When the first driver, Bill Shannon, arrived at his checkpoint in Tolovana on January 28th, his nose was black with frostbite, and three of his dogs had died. The driver who relieved Bill Shannon, named Edgar Kalland, needed the owner of a local roadhouse to pour hot water over his hands to free them from the sled’s metal handlebar. Two more dogs from another relay team died before the serum was passed to Seppala at a town called Ungalik.
THE FINAL STRETCHES
Seppala and his team raced across the ice of the Norton Sound in the dead of night on January 31, with wind chill temperatures nearing an astonishing -90 degrees. The team traveled 84 miles in a single day before stopping to rest – and once rested, they set off again in the middle of the night through a raging winter storm. The team made it across the ice, as well as a 5,000-foot ascent up Little McKinley Mountain, to pass the serum to another driver in record time. The serum was now just 78 miles from Nome, and the death toll in town had reached 28.
The serum reached Gunnar Kaasen and his team of dogs on February 1st. Balto, Kaasen’s lead dog, guided the team heroically through a winter storm that was so severe Kaasen later reported not being able to see the dogs that were just a few feet ahead of him.
Visibility was so poor, in fact, that Kaasen ran his sled two miles past the relay point before noticing – and not wanting to lose a minute, he decided to forge on ahead rather than doubling back to deliver the serum to another driver. As they continued through the storm, the hurricane-force winds ripped past Kaasen’s sled at one point and toppled the sled – and the serum – overboard. The cylinder containing the antitoxin was left buried in the snow – and Kaasen tore off his gloves and dug through the tundra to locate it. Though it resulted in a bad case of frostbite, Kaasen eventually found the cylinder and kept driving.
Kaasen arrived at the next relay point on February 2nd, hours ahead of schedule. When he got there, however, he found the relay driver of the next team asleep. Kaasen took a risk and decided not to wake him, fearing that time would be wasted with the next driver readying his team. Kaasen, Balto, and the rest of the team forged on, driving another 25 miles before finally reaching Nome just before six in the morning. Eyewitnesses described Kaasen pulling up to the town’s bank and stumbling to the front of the sled. There, he collapsed in exhaustion, telling onlookers that Balto was “a damn fine dog.”
A LIVING LEGACY
Just a few hours after Balto’s heroic arrival in Nome, the serum had been thawed and was ready to administer to the patients with diphtheria. Amazingly, the relay team managed to complete the entire journey in just 127 hours – a world record at the time – without one serum vial damaged or destroyed. The serum shipment that arrived by dogsled – along with additional serum deliveries that followed in the next several weeks – were successful in stopping the outbreak in its tracks.
Balto and several other dogs – including Togo, the lead dog on Seppala’s team – were celebrated as local heroes after the race. Balto died in 1933, while the last of the human serum runners died in 1999 – but their legacy lives on: In early 2021, an all-female team of healthcare workers made the news by braving the Alaskan winter to deliver COVID-19 vaccines to people in rural North Alaska, traveling by bobsled and snowmobile – a heroic journey, and one that would have been unthinkable had Balto, Togo, and the 1925 sled runners not first paved the way.
7 Reasons Why We Should Not Need Boosters for COVID-19
A top infectious disease physician explains why immunity derived from natural infection and the vaccines should be long-lasting.
There are at least 7 reasons why immunity after vaccination or infection with COVID-19 should likely be long-lived. If durable, I do not think boosters will be necessary in the future, despite CEOs of pharmaceutical companies (who stand to profit from boosters) messaging that they may and readying such boosters. To explain these reasons, let's orient ourselves to the main components of the immune system.
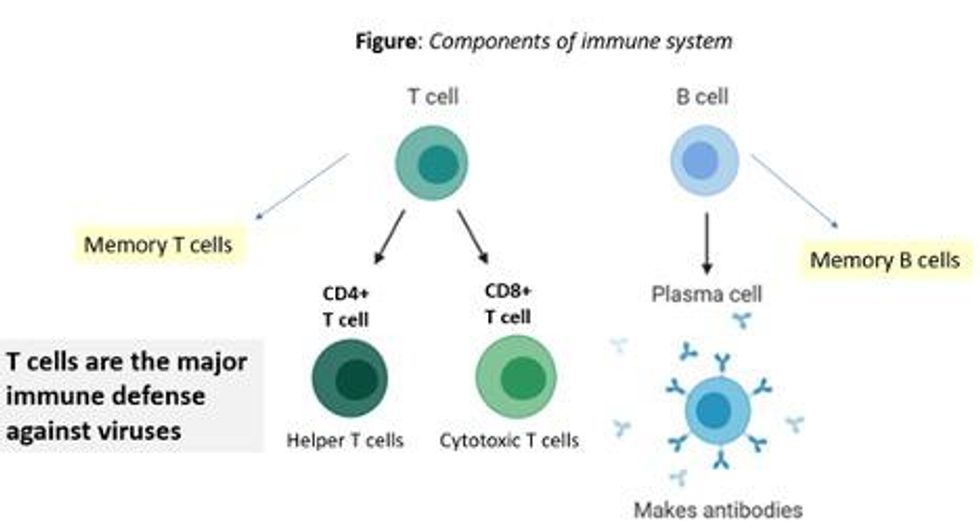
There are two major arms of the immune system: B cells (which produce antibodies) and T cells (which are formed specifically to attack and kill pathogens). T cells are divided into two types, CD4 cells ("helper" T cells) and CD8 cells ("cytotoxic" T cells).
Each arm, once stimulated by infection or vaccine, should hopefully make "memory" banks. So if the body sees the pathogen in the future, these defenses should come roaring back to attack the virus and protect you from getting sick. Plenty of research in COVID-19 indicates a likely long-lasting response to the vaccine or infection. Here are seven of the most compelling reasons:
REASON 1: Memory B Cells Are Produced By Vaccines and Natural Infection
In one study, 12 volunteers who had never had Covid-19--and were fully vaccinated with two Pfizer/BioNTech shots-- underwent biopsies of their lymph nodes. This is where memory B cells are stored in places called "germinal centers". The biopsies were performed three, four, six, and seven weeks after the first mRNA vaccine shot, and were stained to reveal that germinal center memory B cells in the lymph nodes increased in concentration over time.
Natural infection also generates memory B cells. Even after antibody levels wane over time, strong memory B cells were detected in the blood of individuals six and eight months after infection in different studies. Indeed, the half-lives of the memory B cells seen in the study examining patients 8 months after COVID-19 led the authors to conclude that "B cell memory to SARS-CoV-2 was robust and is likely long-lasting." Reason #2 tells us that memory B cells can be active for a very long time indeed.
REASON #2: Memory B Cells Can Produce Neutralizing Antibodies If They See Infection Again Decades Later
Demonstrated production of memory B cells after vaccination or natural infection with COVID-19 is so important because memory B cells, once generated, can be activated to produce high levels of neutralizing antibodies against the pathogen even if encountered many years after the initial exposure. In one amazing study (published in 2008), researchers isolated memory B cells against the 1918 flu strain from the blood of 32 individuals aged 91-101 years. These people had been born on or before 1915 and had survived that pandemic.
Their memory B cells, when exposed to the 1918 flu strain in a test tube, generated high levels of neutralizing antibodies against the virus -- antibodies that then protected mice from lethal infection with this deadly strain. The ability of memory B cells to produce complex antibody responses against an infection nine decades after exposure speaks to their durability.
REASON #3: Vaccines or Natural Infection Trigger Strong Memory T Cell Immunity
All of the trials of the major COVID-19 vaccine candidates measured strong T cell immunity following vaccination, most often assessed by measuring SARS-CoV-2 specific T cells in the phase I/II safety and immunogenicity studies. There are a number of studies that demonstrate the production of strong T cell immunity to COVID-19 after natural infection as well, even when the infection was mild or asymptomatic.
The same study that showed us robust memory B cell production 8 months after natural infection also demonstrated strong and sustained memory T cell production. In fact, the half-lives of the memory T cells in this cohort were long (~125-225 days for CD8+ and ~94-153 days for CD4+ T cells), comparable to the 123-day half-life observed for memory CD8+ T cells after yellow fever immunization (a vaccine usually given once over a lifetime).
A recent study of individuals recovered from COVID-19 show that the initial T cells generated by natural infection mature and differentiate over time into memory T cells that will be "put in the bank" for sustained periods.
REASON #4: T Cell Immunity Following Vaccinations for Other Infections Is Long-Lasting
Last year, we were fortunate to be able to measure how T cell immunity is generated by COVID-19 vaccines, which was not possible in earlier eras when vaccine trials were done for other infections (such as measles, mumps, rubella, pertussis, diphtheria). Antibodies are just the "tip of the iceberg" when assessing the response to vaccination, but were the only arm of the immune response that could be measured following vaccination in the past.
Measuring pathogen-specific T cell responses takes sophisticated technology. However, T cell responses, when assessed years after vaccination for other pathogens, has been shown to be long-lasting. For example, in one study of 56 volunteers who had undergone measles vaccination when they were much younger, strong CD8 and CD4 cell responses to vaccination could be detected up to 34 years later.
REASON #5: T Cell Immunity to Related Coronaviruses That Caused Severe Disease is Long-Lasting
SARS-CoV-2 is a coronavirus that causes severe disease, unlike coronaviruses that cause the common cold. Two other coronaviruses in the recent past caused severe disease, specifically Severely Acute Respiratory Distress Syndrome (SARS) in late 2002-2003 and Middle East Respiratory Syndrome (MERS) in 2011.
A study performed in 2020 demonstrated that the blood of 23 recovered SARS patients possess long-lasting memory T cells that were still reactive to SARS 17 years after the outbreak in 2003. Many scientists expect that T cell immunity to SARS-CoV-2 will be equally durable to that of its cousin.
REASON #6: T Cell Responses from Vaccination and Natural Infection With the Ancestral Strain of COVID-19 Are Robust Against Variants
Even though antibody responses from vaccination may be slightly lower against various COVID-19 variants of concern that have emerged in recent months, T cell immunity after vaccination has been shown to be unperturbed by mutations in the spike protein (in the variants). For instance, T cell responses after mRNA vaccines maintained strong activity against different variants (including P.1 Brazil variant, B.1.1.7 UK variant, B.1.351 South Africa variant and the CA.20.C California variant) in a recent study.
Another study showed that the vaccines generated robust T cell immunity that was unfazed by different variants, including B.1.351 and B.1.1.7. The CD4 and CD8 responses generated after natural infection are equally robust, showing activity against multiple "epitopes" (little segments) of the spike protein of the virus. For instance, CD8 cells responds to 52 epitopes and CD4 cells respond to 57 epitopes across the spike protein, so that a few mutations in the variants cannot knock out such a robust and in-breadth T cell response. Indeed, a recent paper showed that mRNA vaccines were 97.4 percent effective against severe COVID-19 disease in Qatar, even when the majority of circulating virus there was from variants of concern (B.1.351 and B.1.1.7).
REASON #7: Coronaviruses Don't Mutate Quickly Like Influenza, Which Requires Annual Booster Shots
Coronaviruses are RNA viruses, like influenza and HIV (which is actually a retrovirus), but do not mutate as quickly as either one. The reason that coronaviruses don't mutate very rapidly is that their replicating mechanism (polymerase) has a strong proofreading mechanism: If the virus mutates, it usually goes back and self-corrects. Mutations can arise with high rates of replication when transmission is very frequent -- as has been seen in recent months with the emergence of SARS-CoV-2 variants during surges. However, the COVID-19 virus will not be mutating like this when we tamp down transmission with mass vaccination.
In conclusion, I and many of my infectious disease colleagues expect the immunity from natural infection or vaccination to COVID-19 to be durable. Let's put discussion of boosters aside and work hard on global vaccine equity and distribution since the pandemic is not over until it is over for us all.
Professor Tim Caulfield stops by the "Making Sense of Science" podcast this month to discuss the infodemic around COVID-19 and how to deal with it.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
Hear the 30-second trailer:
Listen to the whole episode:
.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

