Why Don’t We Have Artificial Wombs for Premature Infants?
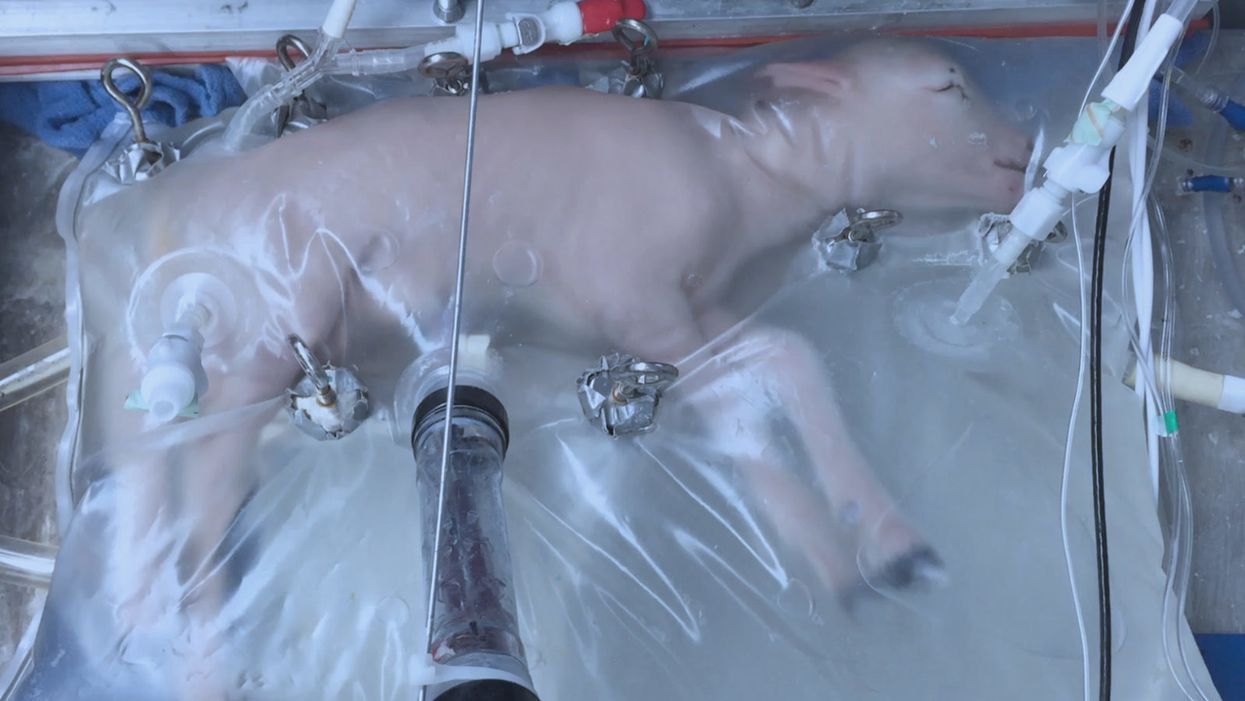
A lamb which was prematurely born at the equivalent of 23 weeks' human gestation, after 28 days of support from an artificial womb.
Ectogenesis, the development of a baby outside of the mother's body, is a concept that dates back to 1923. That year, British biochemist-geneticist J.B.S. Haldane gave a lecture to the "Heretics Society" of the University of Cambridge in which he predicted the invention of an artificial womb by 1960, leading to 70 percent of newborns being born that way by the 2070s. In reality, that's about when an artificial womb could be clinically operational, but trends in science and medicine suggest that such technology would come in increments, each fraught with ethical and social challenges.
An extra-uterine support device could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants.
Currently, one major step is in the works, a system called an extra-uterine support device (EUSD) –or sometimes Ex-Vivo uterine Environment (EVE)– which researchers at the Children's Hospital of Philadelphia have been using to support fetal lambs outside the mother. It also has been called an artificial placenta, because it supplies nutrient- and oxygen-rich blood to the developing lambs via the umbilical vein and receives blood full of waste products through the umbilical arteries. It does not do everything that a natural placenta does, yet it does do some things that a placenta doesn't do. It breathes for the fetus like the mother's lungs, and encloses the fetus in sterile fluid, just like the amniotic sac. It represents a solution to one set of technical challenges in the path to an artificial womb, namely how to keep oxygen flowing into a fetus and carbon dioxide flowing out when the fetal lungs are not ready to function.
Capable of supporting fetal lambs physiologically equivalent to a human fetus at 23 weeks' gestation or earlier, the EUSD could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants. Existing medical technology can keep human infants alive when born in this 23-week range, or even slightly less —the record is just below 22 weeks. But survival is low, because most of the treatment is directed at the lungs, the last major body system to mature to a functional status. This leads to complications not only in babies born before 24 weeks' gestation, but also in a fairly large number of births up to 28 weeks' gestation.
So, the EUSD is basically an advanced neonatal life support machine that beckons to square off the survival curve for infants born up to the 28th week. That is no doubt a good thing, but given the political prominence of reproductive issues, might any societal obstacles be looming?
"While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount."
Health care attorney and clinical ethicist David N. Hoffman points out that even though the EUSD may shift the concept of fetal viability away from the maturity of developing lungs, it would not change the current relationship of the fetus to the mother during pregnancy.
"Our social and legal frameworks, including Roe v. Wade, invite the view of the embryo-fetus as resembling a parasite. Not in a negative sense, but functionally, since it obtains its life support from the mother, while she does not need the fetus for her own physical health," notes Hoffman, who holds faculty appointments at Columbia University, and at the Benjamin N. Cardozo School of Law and the Albert Einstein College of Medicine, of Yeshiva University. "In contrast, our ethical conception of the relationship is grounded in the nurturing responsibility of parenthood. We prioritize the welfare of both mother and fetus ethically, but we lean toward the side of the mother's legal rights, regarding her health throughout pregnancy, and her right to control her womb for most of pregnancy. While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount, on the basis of traditional notions of personhood and parenthood."
Outside of legal frameworks, religion, of course, is a major factor in how society reacts to new reproductive technologies, and an artificial womb would trigger a spectrum of responses.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity."
Speaking from the perspective of Lutheran scholarship, Dr. Daniel Deen, Assistant Professor of Philosophy at Concordia University in Irvine, Calif., does not foresee any objections to the EUSD, either theologically, or generally from Lutherans (who tend to be conservative on reproductive issues), since the EUSD is basically an improvement on current management of prematurity. But things would change with the advent of a full-blown artificial womb.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity," says Deen, who specializes in the philosophy of science. "They may see the artificial womb as a catalyst for strengthening the mechanistic view of reproduction that dominates the thinking of secular society, and of other religious groups, including more liberal Christians."
Judaism, however, appears to be more receptive, even during the research phases.
"Even if researchers strive for a next-generation EUSD aimed at supporting a fetus several weeks earlier than possible with the current system, it still keeps the fetus inside the mother well beyond the 40-day threshold, so there likely are no concerns in terms of Jewish law," says Kalman Laufer, a rabbinical student and executive director of the Medical Ethics Society at Yeshiva University. Referring to a concept from the Babylonian Talmud that an embryo is "like water" until 40 days into pregnancy, at which time it receives a kind of almost-human status warranting protection, Laufer cautions that he's speaking about artificial wombs developed for the sake of rescuing very premature infants. At the same time though, he expects that artificial womb research will eventually trigger a series of complex, legalistic opinions from Jewish scholars, as biotechnology moves further toward supporting fetal growth entirely outside a woman's body.
"Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it."
While the technology treads into uncomfortable territory for social conservatives at first glance, it's possible that the prospect of taking the abortion debate in a whole new direction could engender support for the artificial womb. "Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it," says Zoltan Istvan, a transhumanist politician and journalist who ran for U.S. president in 2016. To some extent, Deen agrees with Istvan, provided we get to a point when the artificial womb is already a reality.
"The world has a way of moving forward despite the fear of its inhabitants," Deen notes. "If the technology gets developed, I could not see any Christians, liberal or conservative, arguing that people seeking abortion ought not opt for a 'transfer' versus an abortive procedure."
So then how realistic is a full-blown artificial womb? The researchers at the Children's Hospital of Philadelphia have noted various technical difficulties that would come up in any attempt to connect a very young fetus to the EUSD and maintain life. One issue is the small umbilical cord blood vessels that must be connected to the EUSD as fetuses of decreasing gestational age are moved outside the mother. Current procedures might be barely adequate for integrating a human fetus into the device in the 18 -21 week range, but going to lower gestational ages would require new technology and different strategies. It also would require numerous other factors to cover for fetal body systems that mature ahead of the lungs and that the current EUSD system is not designed to replace. However, biotechnology and tissue engineering strategies on the horizon could be added to later EUSDs. To address the blood vessel size issue, artificial womb research could benefit by drawing on experts in microfluidics, the field concerned with manipulation of tiny amounts of fluid through very small spaces, and which is ushering in biotech innovations like the "lab on a chip".
"The artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
If the technical challenges to an artificial womb are indeed overcome, reproductive policy debates could be turned on their side.
"Evolution of the EUSD into a full-blown artificial external uterus has ramifications for any reproductive rights issues where policy currently assumes that a mother is needed for a fertilized egg to become a person," says Hoffman, the ethicist and legal scholar. "If we consider debates over whether to keep cryopreserved human embryos in storage, destroy them, or utilize them for embryonic stem cell research or therapies, the artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
Such a scenario, of course, depends on today's developments not being curtailed or sidetracked by societal objections before full-blown ectogenesis is feasible. But if this does ever become a reality, the history of other biotechnologies suggests that some segment of society will embrace the new innovation and never look back.
Gene Transfer Leads to Longer Life and Healthspan
In August, a study provided the first proof-of-principle that genetic material transferred from one species to another can increase both longevity and healthspan in the recipient animal.
The naked mole rat won’t win any beauty contests, but it could possibly win in the talent category. Its superpower: fighting the aging process to live several times longer than other animals its size, in a state of youthful vigor.
It’s believed that naked mole rats experience all the normal processes of wear and tear over their lifespan, but that they’re exceptionally good at repairing the damage from oxygen free radicals and the DNA errors that accumulate over time. Even though they possess genes that make them vulnerable to cancer, they rarely develop the disease, or any other age-related disease, for that matter. Naked mole rats are known to live for over 40 years without any signs of aging, whereas mice live on average about two years and are highly prone to cancer.
Now, these remarkable animals may be able to share their superpower with other species. In August, a study provided what may be the first proof-of-principle that genetic material transferred from one species can increase both longevity and healthspan in a recipient animal.
There are several theories to explain the naked mole rat’s longevity, but the one explored in the study, published in Nature, is based on the abundance of large-molecule high-molecular mass hyaluronic acid (HMM-HA).
A small molecule version of hyaluronic acid is commonly added to skin moisturizers and cosmetics that are marketed as ways to keep skin youthful, but this version, just applied to the skin, won’t have a dramatic anti-aging effect. The naked mole rat has an abundance of the much-larger molecule, HMM-HA, in the chemical-rich solution between cells throughout its body. But does the HMM-HA actually govern the extraordinary longevity and healthspan of the naked mole rat?
To answer this question, Dr. Vera Gorbunova, a professor of biology and oncology at the University of Rochester, and her team created a mouse model containing the naked mole rat gene hyaluronic acid synthase 2, or nmrHas2. It turned out that the mice receiving this gene during their early developmental stage also expressed HMM-HA.
The researchers found that the effects of the HMM-HA molecule in the mice were marked and diverse, exceeding the expectations of the study’s co-authors. High-molecular mass hyaluronic acid was more abundant in kidneys, muscles and other organs of the Has2 mice compared to control mice.
In addition, the altered mice had a much lower incidence of cancer. Seventy percent of the control mice eventually developed cancer, compared to only 57 percent of the altered mice, even after several techniques were used to induce the disease. The biggest difference occurred in the oldest mice, where the cancer incidence for the Has2 mice and the controls was 47 percent and 83 percent, respectively.
With regard to longevity, Has2 males increased their lifespan by more than 16 percent and the females added 9 percent. “Somehow the effect is much more pronounced in male mice, and we don’t have a perfect answer as to why,” says Dr. Gorbunova. Another improvement was in the healthspan of the altered mice: the number of years they spent in a state of relative youth. There’s a frailty index for mice, which includes body weight, mobility, grip strength, vision and hearing, in addition to overall conditions such as the health of the coat and body temperature. The Has2 mice scored lower in frailty than the controls by all measures. They also performed better in tests of locomotion and coordination, and in bone density.
Gorbunova’s results show that a gene artificially transferred from one species can have a beneficial effect on another species for longevity, something that had never been demonstrated before. This finding is “quite spectacular,” said Steven Austad, a biologist at the University of Alabama at Birmingham, who was not involved in the study.
Just as in lifespan, the effects in various organs and systems varied between the sexes, a common occurrence in longevity research, according to Austad, who authored the book Methuselah’s Zoo and specializes in the biological differences between species. “We have ten drugs that we can give to mice to make them live longer,” he says, “and all of them work better in one sex than in the other.” This suggests that more attention needs to be paid to the different effects of anti-aging strategies between the sexes, as well as gender differences in healthspan.
According to the study authors, the HMM-HA molecule delivered these benefits by reducing inflammation and senescence (cell dysfunction and death). The molecule also caused a variety of other benefits, including an upregulation of genes involved in the function of mitochondria, the powerhouses of the cells. These mechanisms are implicated in the aging process, and in human disease. In humans, virtually all noncommunicable diseases entail an acceleration of the aging process.
So, would the gene that creates HMM-HA have similar benefits for longevity in humans? “We think about these questions a lot,” Gorbunova says. “It’s been done by injections in certain patients, but it has a local effect in the treatment of organs affected by disease,” which could offer some benefits, she added.
“Mice are very short-lived and cancer-prone, and the effects are small,” says Steven Austad, a biologist at the University of Alabama at Birmingham. “But they did live longer and stay healthy longer, which is remarkable.”
As for a gene therapy to introduce the nmrHas2 gene into humans to obtain a global result, she’s skeptical because of the complexity involved. Gorbunova notes that there are potential dangers in introducing an animal gene into humans, such as immune responses or allergic reactions.
Austad is equally cautious about a gene therapy. “What this study says is that you can take something a species does well and transfer at least some of that into a new species. It opens up the way, but you may need to transfer six or eight or ten genes into a human” to get the large effect desired. Humans are much more complex and contain many more genes than mice, and all systems in a biological organism are intricately connected. One naked mole rat gene may not make a big difference when it interacts with human genes, metabolism and physiology.
Still, Austad thinks the possibilities are tantalizing. “Mice are very short-lived and cancer-prone, and the effects are small,” he says. “But they did live longer and stay healthy longer, which is remarkable.”
As for further research, says Austad, “The first place to look is the skin” to see if the nmrHas2 gene and the HMM-HA it produces can reduce the chance of cancer. Austad added that it would be straightforward to use the gene to try to prevent cancer in skin cells in a dish to see if it prevents cancer. It would not be hard to do. “We don’t know of any downsides to hyaluronic acid in skin, because it’s already used in skin products, and you could look at this fairly quickly.”
“Aging mechanisms evolved over a long time,” says Gorbunova, “so in aging there are multiple mechanisms working together that affect each other.” All of these processes could play a part and almost certainly differ from one species to the next.
“HMM-HA molecules are large, but we’re now looking for a small-molecule drug that would slow it’s breakdown,” she says. “And we’re looking for inhibitors, now being tested in mice, that would hinder the breakdown of hyaluronic acid.” Gorbunova has found a natural, plant-based product that acts as an inhibitor and could potentially be taken as a supplement. Ultimately, though, she thinks that drug development will be the safest and most effective approach to delivering HMM-HA for anti-aging.
A new study provides key insights in what causes Alzheimer's: a breakdown in the brain’s system for clearing waste.
In recent years, researchers of Alzheimer’s have made progress in figuring out the complex factors that lead to the disease. Yet, the root cause, or causes, of Alzheimer’s are still pretty much a mystery.
In fact, many people get Alzheimer’s even though they lack the gene variant we know can play a role in the disease. This is a critical knowledge gap for research to address because the vast majority of Alzheimer’s patients don’t have this variant.
A new study provides key insights into what’s causing the disease. The research, published in Nature Communications, points to a breakdown over time in the brain’s system for clearing waste, an issue that seems to happen in some people as they get older.
Michael Glickman, a biologist at Technion – Israel Institute of Technology, helped lead this research. I asked him to tell me about his approach to studying how this breakdown occurs in the brain, and how he tested a treatment that has potential to fix the problem at its earliest stages.
Dr. Michael Glickman is internationally renowned for his research on the ubiquitin-proteasome system (UPS), the brain's system for clearing the waste that is involved in diseases such as Huntington's, Alzheimer's, and Parkinson's. He is the head of the Lab for Protein Characterization in the Faculty of Biology at the Technion – Israel Institute of Technology. In the lab, Michael and his team focus on protein recycling and the ubiquitin-proteasome system, which protects against serious diseases like Alzheimer’s, Parkinson’s, cystic fibrosis, and diabetes. After earning his PhD at the University of California at Berkeley in 1994, Michael joined the Technion as a Senior Lecturer in 1998 and has served as a full professor since 2009.

Dr. Michael Glickman

