Why Don’t We Have Artificial Wombs for Premature Infants?
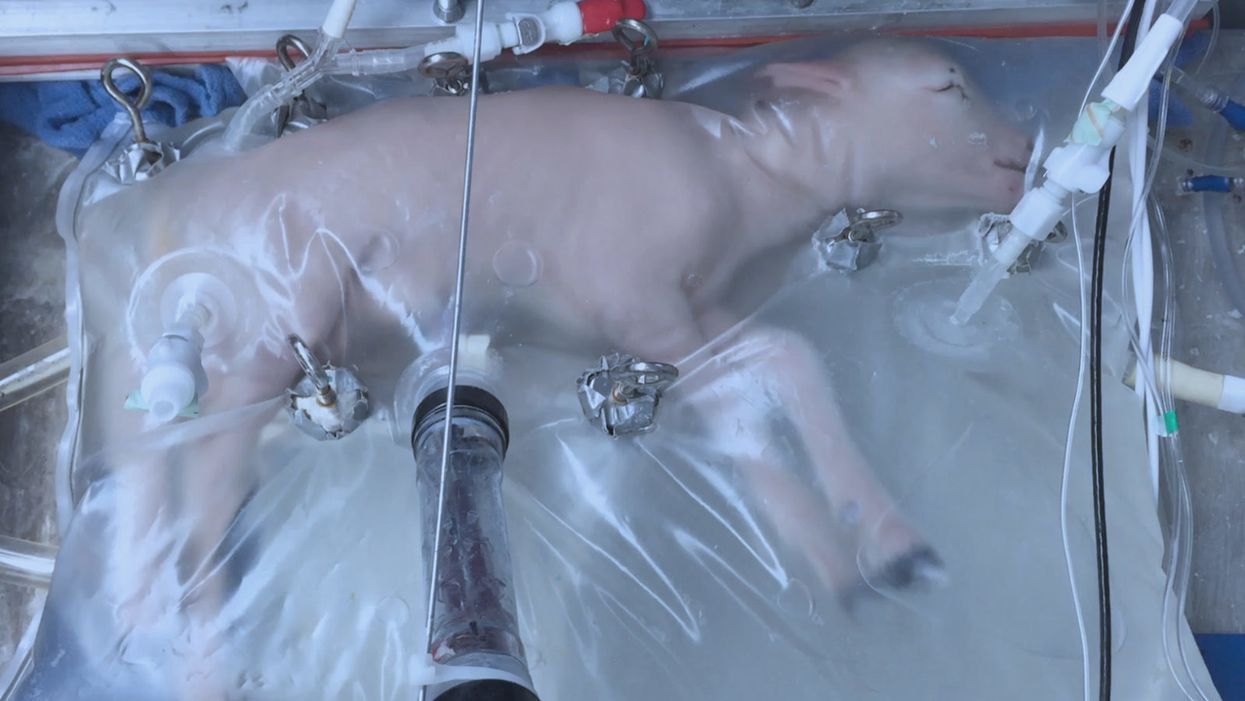
A lamb which was prematurely born at the equivalent of 23 weeks' human gestation, after 28 days of support from an artificial womb.
Ectogenesis, the development of a baby outside of the mother's body, is a concept that dates back to 1923. That year, British biochemist-geneticist J.B.S. Haldane gave a lecture to the "Heretics Society" of the University of Cambridge in which he predicted the invention of an artificial womb by 1960, leading to 70 percent of newborns being born that way by the 2070s. In reality, that's about when an artificial womb could be clinically operational, but trends in science and medicine suggest that such technology would come in increments, each fraught with ethical and social challenges.
An extra-uterine support device could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants.
Currently, one major step is in the works, a system called an extra-uterine support device (EUSD) –or sometimes Ex-Vivo uterine Environment (EVE)– which researchers at the Children's Hospital of Philadelphia have been using to support fetal lambs outside the mother. It also has been called an artificial placenta, because it supplies nutrient- and oxygen-rich blood to the developing lambs via the umbilical vein and receives blood full of waste products through the umbilical arteries. It does not do everything that a natural placenta does, yet it does do some things that a placenta doesn't do. It breathes for the fetus like the mother's lungs, and encloses the fetus in sterile fluid, just like the amniotic sac. It represents a solution to one set of technical challenges in the path to an artificial womb, namely how to keep oxygen flowing into a fetus and carbon dioxide flowing out when the fetal lungs are not ready to function.
Capable of supporting fetal lambs physiologically equivalent to a human fetus at 23 weeks' gestation or earlier, the EUSD could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants. Existing medical technology can keep human infants alive when born in this 23-week range, or even slightly less —the record is just below 22 weeks. But survival is low, because most of the treatment is directed at the lungs, the last major body system to mature to a functional status. This leads to complications not only in babies born before 24 weeks' gestation, but also in a fairly large number of births up to 28 weeks' gestation.
So, the EUSD is basically an advanced neonatal life support machine that beckons to square off the survival curve for infants born up to the 28th week. That is no doubt a good thing, but given the political prominence of reproductive issues, might any societal obstacles be looming?
"While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount."
Health care attorney and clinical ethicist David N. Hoffman points out that even though the EUSD may shift the concept of fetal viability away from the maturity of developing lungs, it would not change the current relationship of the fetus to the mother during pregnancy.
"Our social and legal frameworks, including Roe v. Wade, invite the view of the embryo-fetus as resembling a parasite. Not in a negative sense, but functionally, since it obtains its life support from the mother, while she does not need the fetus for her own physical health," notes Hoffman, who holds faculty appointments at Columbia University, and at the Benjamin N. Cardozo School of Law and the Albert Einstein College of Medicine, of Yeshiva University. "In contrast, our ethical conception of the relationship is grounded in the nurturing responsibility of parenthood. We prioritize the welfare of both mother and fetus ethically, but we lean toward the side of the mother's legal rights, regarding her health throughout pregnancy, and her right to control her womb for most of pregnancy. While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount, on the basis of traditional notions of personhood and parenthood."
Outside of legal frameworks, religion, of course, is a major factor in how society reacts to new reproductive technologies, and an artificial womb would trigger a spectrum of responses.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity."
Speaking from the perspective of Lutheran scholarship, Dr. Daniel Deen, Assistant Professor of Philosophy at Concordia University in Irvine, Calif., does not foresee any objections to the EUSD, either theologically, or generally from Lutherans (who tend to be conservative on reproductive issues), since the EUSD is basically an improvement on current management of prematurity. But things would change with the advent of a full-blown artificial womb.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity," says Deen, who specializes in the philosophy of science. "They may see the artificial womb as a catalyst for strengthening the mechanistic view of reproduction that dominates the thinking of secular society, and of other religious groups, including more liberal Christians."
Judaism, however, appears to be more receptive, even during the research phases.
"Even if researchers strive for a next-generation EUSD aimed at supporting a fetus several weeks earlier than possible with the current system, it still keeps the fetus inside the mother well beyond the 40-day threshold, so there likely are no concerns in terms of Jewish law," says Kalman Laufer, a rabbinical student and executive director of the Medical Ethics Society at Yeshiva University. Referring to a concept from the Babylonian Talmud that an embryo is "like water" until 40 days into pregnancy, at which time it receives a kind of almost-human status warranting protection, Laufer cautions that he's speaking about artificial wombs developed for the sake of rescuing very premature infants. At the same time though, he expects that artificial womb research will eventually trigger a series of complex, legalistic opinions from Jewish scholars, as biotechnology moves further toward supporting fetal growth entirely outside a woman's body.
"Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it."
While the technology treads into uncomfortable territory for social conservatives at first glance, it's possible that the prospect of taking the abortion debate in a whole new direction could engender support for the artificial womb. "Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it," says Zoltan Istvan, a transhumanist politician and journalist who ran for U.S. president in 2016. To some extent, Deen agrees with Istvan, provided we get to a point when the artificial womb is already a reality.
"The world has a way of moving forward despite the fear of its inhabitants," Deen notes. "If the technology gets developed, I could not see any Christians, liberal or conservative, arguing that people seeking abortion ought not opt for a 'transfer' versus an abortive procedure."
So then how realistic is a full-blown artificial womb? The researchers at the Children's Hospital of Philadelphia have noted various technical difficulties that would come up in any attempt to connect a very young fetus to the EUSD and maintain life. One issue is the small umbilical cord blood vessels that must be connected to the EUSD as fetuses of decreasing gestational age are moved outside the mother. Current procedures might be barely adequate for integrating a human fetus into the device in the 18 -21 week range, but going to lower gestational ages would require new technology and different strategies. It also would require numerous other factors to cover for fetal body systems that mature ahead of the lungs and that the current EUSD system is not designed to replace. However, biotechnology and tissue engineering strategies on the horizon could be added to later EUSDs. To address the blood vessel size issue, artificial womb research could benefit by drawing on experts in microfluidics, the field concerned with manipulation of tiny amounts of fluid through very small spaces, and which is ushering in biotech innovations like the "lab on a chip".
"The artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
If the technical challenges to an artificial womb are indeed overcome, reproductive policy debates could be turned on their side.
"Evolution of the EUSD into a full-blown artificial external uterus has ramifications for any reproductive rights issues where policy currently assumes that a mother is needed for a fertilized egg to become a person," says Hoffman, the ethicist and legal scholar. "If we consider debates over whether to keep cryopreserved human embryos in storage, destroy them, or utilize them for embryonic stem cell research or therapies, the artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
Such a scenario, of course, depends on today's developments not being curtailed or sidetracked by societal objections before full-blown ectogenesis is feasible. But if this does ever become a reality, the history of other biotechnologies suggests that some segment of society will embrace the new innovation and never look back.
A startup aims to make medicines in space
A company is looking to improve medicines by making them in the nearly weightless environment of space.
Story by Big Think
On June 12, a SpaceX Falcon 9 rocket deployed 72 small satellites for customers — including the world’s first space factory.
The challenge: In 2019, pharma giant Merck revealed that an experiment on the International Space Station had shown how to make its blockbuster cancer drug Keytruda more stable. That meant it could now be administered via a shot rather than through an IV infusion.
The key to the discovery was the fact that particles behave differently when freed from the force of gravity — seeing how its drug crystalized in microgravity helped Merck figure out how to tweak its manufacturing process on Earth to produce the more stable version.
Microgravity research could potentially lead to many more discoveries like this one, or even the development of brand-new drugs, but ISS astronauts only have so much time for commercial experiments.
“There are many high-performance products that are only possible to make in zero-gravity, which is a manufacturing capability that cannot be replicated in any factory on Earth.”-- Will Bruey.
The only options for accessing microgravity (or free fall) outside of orbit, meanwhile, are parabolic airplane flights and drop towers, and those are only useful for experiments that require less than a minute in microgravity — Merck’s ISS experiment took 18 days.
The idea: In 2021, California startup Varda Space Industries announced its intention to build the world’s first space factory, to manufacture not only pharmaceuticals but other products that could benefit from being made in microgravity, such as semiconductors and fiber optic cables.
This factory would consist of a commercial satellite platform attached to two Varda-made modules. One module would contain equipment capable of autonomously manufacturing a product. The other would be a reentry capsule to bring the finished goods back to Earth.
“There are many high-performance products that are only possible to make in zero-gravity, which is a manufacturing capability that cannot be replicated in any factory on Earth,” said CEO Will Bruey, who’d previously developed and flown spacecraft for SpaceX.
“We have a team stacked with aerospace talent in the prime of their careers, focused on getting working hardware to orbit as quickly as possible,” he continued.
“[Pharmaceuticals] are the most valuable chemicals per unit mass. And they also have a large market on Earth.” -- Will Bruey, CEO of Varda Space.
What’s new? At the time, Varda said it planned to launch its first space factory in 2023, and, in what feels like a first for a space startup, it has actually hit that ambitious launch schedule.
“We have ACQUISITION OF SIGNAL,” the startup tweeted soon after the Falcon 9 launch on June 12. “The world’s first space factory’s solar panels have found the sun and it’s beginning to de-tumble.”
During the satellite’s first week in space, Varda will focus on testing its systems to make sure everything works as hoped. The second week will be dedicated to heating and cooling the old HIV-AIDS drug ritonavir repeatedly to study how its particles crystalize in microgravity.
After about a month in space, Varda will attempt to bring its first space factory back to Earth, sending it through the atmosphere at hypersonic speeds and then using a parachute system to safely land at the Department of Defense’s Utah Test and Training Range.
Looking ahead: Ultimately, Varda’s space factories could end up serving dual purposes as manufacturing facilities and hypersonic testbeds — the Air Force has already awarded the startup a contract to use its next reentry capsule to test hardware for hypersonic missiles.
But as for manufacturing other types of goods, Varda plans to stick with drugs for now.
“[Pharmaceuticals] are the most valuable chemicals per unit mass,” Bruey told CNN. “And they also have a large market on Earth.”
“You’re not going to see Varda do anything other than pharmaceuticals for the next minimum of six, seven years,” added Delian Asparouhov, Varda’s co-founder and president.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Genes that protect health with Dr. Nir Barzilai
Centenarians essentially won the genetic lottery, says Nir Barzilai of Albert Einstein College of Medicine. He is studying their genes to see how the rest of us can benefit from understanding how they work.
In today’s podcast episode, I talk with Nir Barzilai, a geroscientist, which means he studies the biology of aging. Barzilai directs the Institute for Aging Research at the Albert Einstein College of Medicine.
My first question for Dr. Barzilai was: why do we age? And is there anything to be done about it? His answers were encouraging. We can’t live forever, but we have some control over the process, as he argues in his book, Age Later.
Dr. Barzilai told me that centenarians differ from the rest of us because they have unique gene mutations that help them stay healthy longer. For most of us, the words “gene mutations” spell trouble - we associate these words with cancer or neurodegenerative diseases, but apparently not all mutations are bad.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Centenarians may have essentially won the genetic lottery, but that doesn’t mean the rest of us are predestined to have a specific lifespan and health span, or the amount of time spent living productively and enjoyably. “Aging is a mother of all diseases,” Dr. Barzilai told me. And as a disease, it can be targeted by therapeutics. Dr. Barzilai’s team is already running clinical trials on such therapeutics — and the results are promising.
More about Dr. Barzilai: He is scientific director of AFAR, American Federation for Aging Research. As part of his work, Dr. Barzilai studies families of centenarians and their genetics to learn how the rest of us can learn and benefit from their super-aging. He also organizing a clinical trial to test a specific drug that may slow aging.
Show Links
Age Later: Health Span, Life Span, and the New Science of Longevity https://www.amazon.com/Age-Later-Healthiest-Sharpest-Centenarians/dp/1250230853
American Federation for Aging Research https://www.afar.org
https://www.afar.org/nir-barzilai
https://www.einsteinmed.edu/faculty/484/nir-barzilai/
Metformin as a Tool to Target Aging
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5943638/
Benefits of Metformin in Attenuating the Hallmarks of Aging https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7347426/
The Longevity Genes Project https://www.einsteinmed.edu/centers/aging/longevity-genes-project/
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.