Why Don’t We Have Artificial Wombs for Premature Infants?
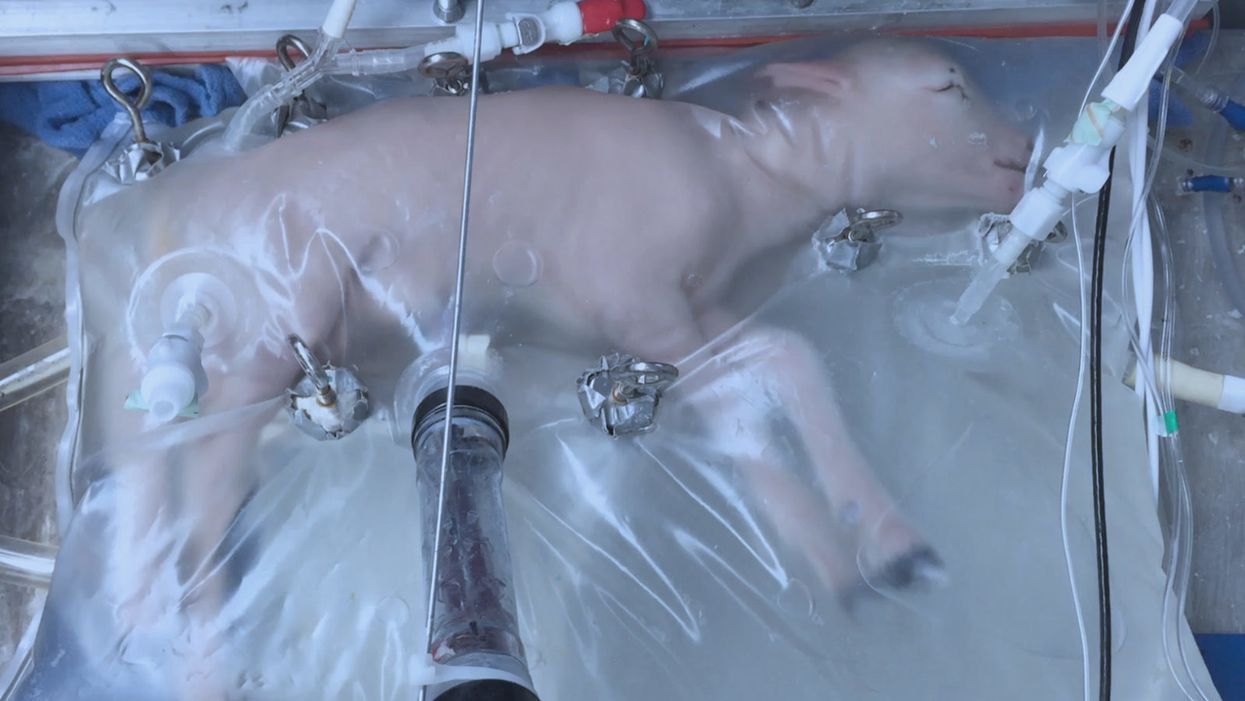
A lamb which was prematurely born at the equivalent of 23 weeks' human gestation, after 28 days of support from an artificial womb.
Ectogenesis, the development of a baby outside of the mother's body, is a concept that dates back to 1923. That year, British biochemist-geneticist J.B.S. Haldane gave a lecture to the "Heretics Society" of the University of Cambridge in which he predicted the invention of an artificial womb by 1960, leading to 70 percent of newborns being born that way by the 2070s. In reality, that's about when an artificial womb could be clinically operational, but trends in science and medicine suggest that such technology would come in increments, each fraught with ethical and social challenges.
An extra-uterine support device could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants.
Currently, one major step is in the works, a system called an extra-uterine support device (EUSD) –or sometimes Ex-Vivo uterine Environment (EVE)– which researchers at the Children's Hospital of Philadelphia have been using to support fetal lambs outside the mother. It also has been called an artificial placenta, because it supplies nutrient- and oxygen-rich blood to the developing lambs via the umbilical vein and receives blood full of waste products through the umbilical arteries. It does not do everything that a natural placenta does, yet it does do some things that a placenta doesn't do. It breathes for the fetus like the mother's lungs, and encloses the fetus in sterile fluid, just like the amniotic sac. It represents a solution to one set of technical challenges in the path to an artificial womb, namely how to keep oxygen flowing into a fetus and carbon dioxide flowing out when the fetal lungs are not ready to function.
Capable of supporting fetal lambs physiologically equivalent to a human fetus at 23 weeks' gestation or earlier, the EUSD could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants. Existing medical technology can keep human infants alive when born in this 23-week range, or even slightly less —the record is just below 22 weeks. But survival is low, because most of the treatment is directed at the lungs, the last major body system to mature to a functional status. This leads to complications not only in babies born before 24 weeks' gestation, but also in a fairly large number of births up to 28 weeks' gestation.
So, the EUSD is basically an advanced neonatal life support machine that beckons to square off the survival curve for infants born up to the 28th week. That is no doubt a good thing, but given the political prominence of reproductive issues, might any societal obstacles be looming?
"While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount."
Health care attorney and clinical ethicist David N. Hoffman points out that even though the EUSD may shift the concept of fetal viability away from the maturity of developing lungs, it would not change the current relationship of the fetus to the mother during pregnancy.
"Our social and legal frameworks, including Roe v. Wade, invite the view of the embryo-fetus as resembling a parasite. Not in a negative sense, but functionally, since it obtains its life support from the mother, while she does not need the fetus for her own physical health," notes Hoffman, who holds faculty appointments at Columbia University, and at the Benjamin N. Cardozo School of Law and the Albert Einstein College of Medicine, of Yeshiva University. "In contrast, our ethical conception of the relationship is grounded in the nurturing responsibility of parenthood. We prioritize the welfare of both mother and fetus ethically, but we lean toward the side of the mother's legal rights, regarding her health throughout pregnancy, and her right to control her womb for most of pregnancy. While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount, on the basis of traditional notions of personhood and parenthood."
Outside of legal frameworks, religion, of course, is a major factor in how society reacts to new reproductive technologies, and an artificial womb would trigger a spectrum of responses.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity."
Speaking from the perspective of Lutheran scholarship, Dr. Daniel Deen, Assistant Professor of Philosophy at Concordia University in Irvine, Calif., does not foresee any objections to the EUSD, either theologically, or generally from Lutherans (who tend to be conservative on reproductive issues), since the EUSD is basically an improvement on current management of prematurity. But things would change with the advent of a full-blown artificial womb.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity," says Deen, who specializes in the philosophy of science. "They may see the artificial womb as a catalyst for strengthening the mechanistic view of reproduction that dominates the thinking of secular society, and of other religious groups, including more liberal Christians."
Judaism, however, appears to be more receptive, even during the research phases.
"Even if researchers strive for a next-generation EUSD aimed at supporting a fetus several weeks earlier than possible with the current system, it still keeps the fetus inside the mother well beyond the 40-day threshold, so there likely are no concerns in terms of Jewish law," says Kalman Laufer, a rabbinical student and executive director of the Medical Ethics Society at Yeshiva University. Referring to a concept from the Babylonian Talmud that an embryo is "like water" until 40 days into pregnancy, at which time it receives a kind of almost-human status warranting protection, Laufer cautions that he's speaking about artificial wombs developed for the sake of rescuing very premature infants. At the same time though, he expects that artificial womb research will eventually trigger a series of complex, legalistic opinions from Jewish scholars, as biotechnology moves further toward supporting fetal growth entirely outside a woman's body.
"Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it."
While the technology treads into uncomfortable territory for social conservatives at first glance, it's possible that the prospect of taking the abortion debate in a whole new direction could engender support for the artificial womb. "Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it," says Zoltan Istvan, a transhumanist politician and journalist who ran for U.S. president in 2016. To some extent, Deen agrees with Istvan, provided we get to a point when the artificial womb is already a reality.
"The world has a way of moving forward despite the fear of its inhabitants," Deen notes. "If the technology gets developed, I could not see any Christians, liberal or conservative, arguing that people seeking abortion ought not opt for a 'transfer' versus an abortive procedure."
So then how realistic is a full-blown artificial womb? The researchers at the Children's Hospital of Philadelphia have noted various technical difficulties that would come up in any attempt to connect a very young fetus to the EUSD and maintain life. One issue is the small umbilical cord blood vessels that must be connected to the EUSD as fetuses of decreasing gestational age are moved outside the mother. Current procedures might be barely adequate for integrating a human fetus into the device in the 18 -21 week range, but going to lower gestational ages would require new technology and different strategies. It also would require numerous other factors to cover for fetal body systems that mature ahead of the lungs and that the current EUSD system is not designed to replace. However, biotechnology and tissue engineering strategies on the horizon could be added to later EUSDs. To address the blood vessel size issue, artificial womb research could benefit by drawing on experts in microfluidics, the field concerned with manipulation of tiny amounts of fluid through very small spaces, and which is ushering in biotech innovations like the "lab on a chip".
"The artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
If the technical challenges to an artificial womb are indeed overcome, reproductive policy debates could be turned on their side.
"Evolution of the EUSD into a full-blown artificial external uterus has ramifications for any reproductive rights issues where policy currently assumes that a mother is needed for a fertilized egg to become a person," says Hoffman, the ethicist and legal scholar. "If we consider debates over whether to keep cryopreserved human embryos in storage, destroy them, or utilize them for embryonic stem cell research or therapies, the artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
Such a scenario, of course, depends on today's developments not being curtailed or sidetracked by societal objections before full-blown ectogenesis is feasible. But if this does ever become a reality, the history of other biotechnologies suggests that some segment of society will embrace the new innovation and never look back.
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
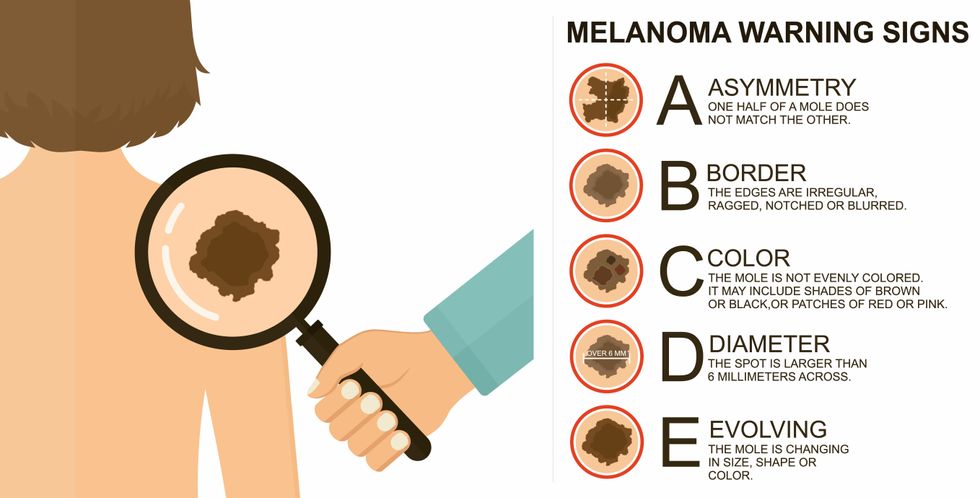
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

