Why Don’t We Have Artificial Wombs for Premature Infants?
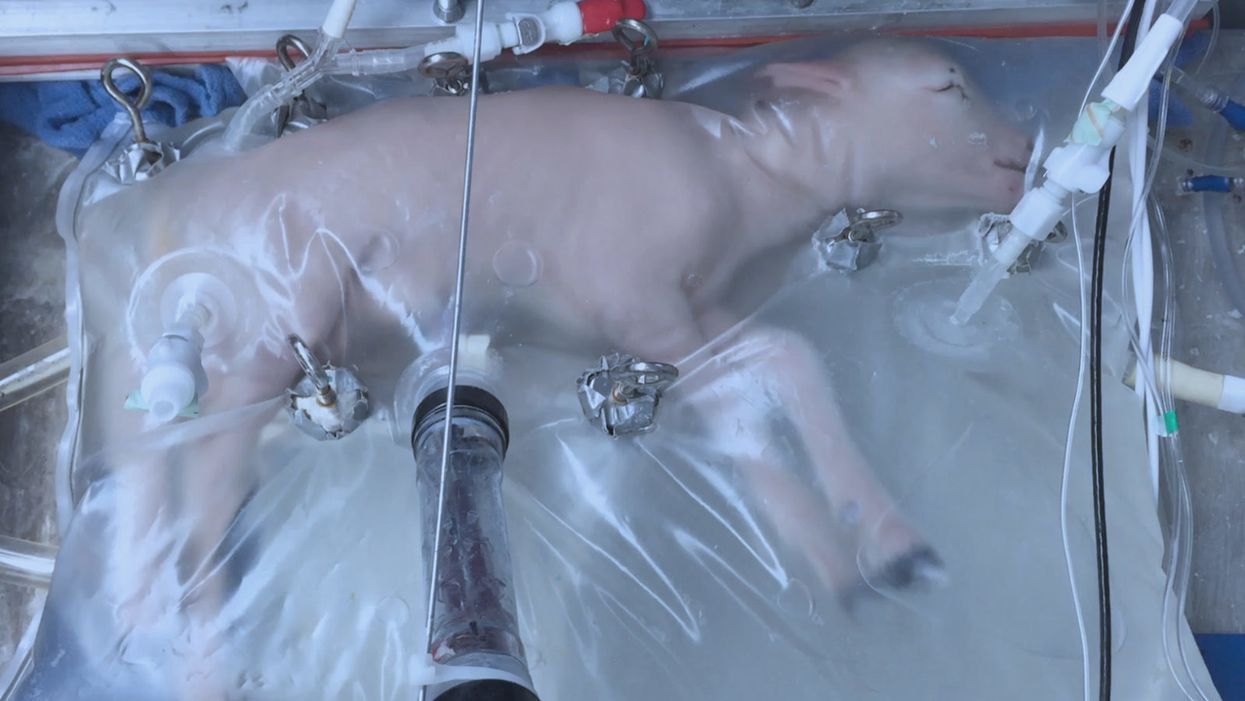
A lamb which was prematurely born at the equivalent of 23 weeks' human gestation, after 28 days of support from an artificial womb.
Ectogenesis, the development of a baby outside of the mother's body, is a concept that dates back to 1923. That year, British biochemist-geneticist J.B.S. Haldane gave a lecture to the "Heretics Society" of the University of Cambridge in which he predicted the invention of an artificial womb by 1960, leading to 70 percent of newborns being born that way by the 2070s. In reality, that's about when an artificial womb could be clinically operational, but trends in science and medicine suggest that such technology would come in increments, each fraught with ethical and social challenges.
An extra-uterine support device could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants.
Currently, one major step is in the works, a system called an extra-uterine support device (EUSD) –or sometimes Ex-Vivo uterine Environment (EVE)– which researchers at the Children's Hospital of Philadelphia have been using to support fetal lambs outside the mother. It also has been called an artificial placenta, because it supplies nutrient- and oxygen-rich blood to the developing lambs via the umbilical vein and receives blood full of waste products through the umbilical arteries. It does not do everything that a natural placenta does, yet it does do some things that a placenta doesn't do. It breathes for the fetus like the mother's lungs, and encloses the fetus in sterile fluid, just like the amniotic sac. It represents a solution to one set of technical challenges in the path to an artificial womb, namely how to keep oxygen flowing into a fetus and carbon dioxide flowing out when the fetal lungs are not ready to function.
Capable of supporting fetal lambs physiologically equivalent to a human fetus at 23 weeks' gestation or earlier, the EUSD could be ready for clinical trials in humans in the next two to four years, with hopes that it could improve survival of very premature infants. Existing medical technology can keep human infants alive when born in this 23-week range, or even slightly less —the record is just below 22 weeks. But survival is low, because most of the treatment is directed at the lungs, the last major body system to mature to a functional status. This leads to complications not only in babies born before 24 weeks' gestation, but also in a fairly large number of births up to 28 weeks' gestation.
So, the EUSD is basically an advanced neonatal life support machine that beckons to square off the survival curve for infants born up to the 28th week. That is no doubt a good thing, but given the political prominence of reproductive issues, might any societal obstacles be looming?
"While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount."
Health care attorney and clinical ethicist David N. Hoffman points out that even though the EUSD may shift the concept of fetal viability away from the maturity of developing lungs, it would not change the current relationship of the fetus to the mother during pregnancy.
"Our social and legal frameworks, including Roe v. Wade, invite the view of the embryo-fetus as resembling a parasite. Not in a negative sense, but functionally, since it obtains its life support from the mother, while she does not need the fetus for her own physical health," notes Hoffman, who holds faculty appointments at Columbia University, and at the Benjamin N. Cardozo School of Law and the Albert Einstein College of Medicine, of Yeshiva University. "In contrast, our ethical conception of the relationship is grounded in the nurturing responsibility of parenthood. We prioritize the welfare of both mother and fetus ethically, but we lean toward the side of the mother's legal rights, regarding her health throughout pregnancy, and her right to control her womb for most of pregnancy. While some may argue that the EUSD system will shift the definition of viability to a point prior to the maturation of the fetus' lungs, ethical and legal frameworks must still recognize the mother's privacy rights as paramount, on the basis of traditional notions of personhood and parenthood."
Outside of legal frameworks, religion, of course, is a major factor in how society reacts to new reproductive technologies, and an artificial womb would trigger a spectrum of responses.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity."
Speaking from the perspective of Lutheran scholarship, Dr. Daniel Deen, Assistant Professor of Philosophy at Concordia University in Irvine, Calif., does not foresee any objections to the EUSD, either theologically, or generally from Lutherans (who tend to be conservative on reproductive issues), since the EUSD is basically an improvement on current management of prematurity. But things would change with the advent of a full-blown artificial womb.
"Significant numbers of conservative Christians may oppose an artificial womb in fear that it might harm the central role of marriage in Christianity," says Deen, who specializes in the philosophy of science. "They may see the artificial womb as a catalyst for strengthening the mechanistic view of reproduction that dominates the thinking of secular society, and of other religious groups, including more liberal Christians."
Judaism, however, appears to be more receptive, even during the research phases.
"Even if researchers strive for a next-generation EUSD aimed at supporting a fetus several weeks earlier than possible with the current system, it still keeps the fetus inside the mother well beyond the 40-day threshold, so there likely are no concerns in terms of Jewish law," says Kalman Laufer, a rabbinical student and executive director of the Medical Ethics Society at Yeshiva University. Referring to a concept from the Babylonian Talmud that an embryo is "like water" until 40 days into pregnancy, at which time it receives a kind of almost-human status warranting protection, Laufer cautions that he's speaking about artificial wombs developed for the sake of rescuing very premature infants. At the same time though, he expects that artificial womb research will eventually trigger a series of complex, legalistic opinions from Jewish scholars, as biotechnology moves further toward supporting fetal growth entirely outside a woman's body.
"Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it."
While the technology treads into uncomfortable territory for social conservatives at first glance, it's possible that the prospect of taking the abortion debate in a whole new direction could engender support for the artificial womb. "Since [the EUSD] gives some justification to end abortion, by transferring fetuses from mother to machine, conservatives will probably rally around it," says Zoltan Istvan, a transhumanist politician and journalist who ran for U.S. president in 2016. To some extent, Deen agrees with Istvan, provided we get to a point when the artificial womb is already a reality.
"The world has a way of moving forward despite the fear of its inhabitants," Deen notes. "If the technology gets developed, I could not see any Christians, liberal or conservative, arguing that people seeking abortion ought not opt for a 'transfer' versus an abortive procedure."
So then how realistic is a full-blown artificial womb? The researchers at the Children's Hospital of Philadelphia have noted various technical difficulties that would come up in any attempt to connect a very young fetus to the EUSD and maintain life. One issue is the small umbilical cord blood vessels that must be connected to the EUSD as fetuses of decreasing gestational age are moved outside the mother. Current procedures might be barely adequate for integrating a human fetus into the device in the 18 -21 week range, but going to lower gestational ages would require new technology and different strategies. It also would require numerous other factors to cover for fetal body systems that mature ahead of the lungs and that the current EUSD system is not designed to replace. However, biotechnology and tissue engineering strategies on the horizon could be added to later EUSDs. To address the blood vessel size issue, artificial womb research could benefit by drawing on experts in microfluidics, the field concerned with manipulation of tiny amounts of fluid through very small spaces, and which is ushering in biotech innovations like the "lab on a chip".
"The artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
If the technical challenges to an artificial womb are indeed overcome, reproductive policy debates could be turned on their side.
"Evolution of the EUSD into a full-blown artificial external uterus has ramifications for any reproductive rights issues where policy currently assumes that a mother is needed for a fertilized egg to become a person," says Hoffman, the ethicist and legal scholar. "If we consider debates over whether to keep cryopreserved human embryos in storage, destroy them, or utilize them for embryonic stem cell research or therapies, the artificial womb might put fathers on equal footing with mothers, since any embryo could potentially achieve personhood without ever seeing the inside of a woman's uterus."
Such a scenario, of course, depends on today's developments not being curtailed or sidetracked by societal objections before full-blown ectogenesis is feasible. But if this does ever become a reality, the history of other biotechnologies suggests that some segment of society will embrace the new innovation and never look back.
Scientists aim to preserve donkeys, one frozen embryo at a time
In Ethiopia, Samuna’s three donkeys help her transport produce to market and to collect the water essential to her family, neighbours and livestock. Donkeys are more endangered than people realize, experts say.
Every day for a week in 2022, Andres Gambini, a veterinarian and senior lecturer in animal science at the University of Queensland in Australia, walked into his lab—and headed straight to the video camera. Trained on an array of about 50 donkey embryos, all created by Gambini’s manual in vitro fertilization, or IVF, the camera kept an eye on their developmental progress. To eventually create a viable embryo that could be implanted into a female donkey, the embryos’ cells had to keep dividing, first in two, then in four and so on.
But the embryos weren’t cooperating. Some would start splitting up only to stop a day or two later, and others wouldn’t start at all. Every day he came in, Gambini saw fewer and fewer dividing embryos, so he was losing faith in the effort. “You see many failed attempts and get disappointed,” he says.
Gambini and his team, a group of Argentinian and Spanish researchers, were working to create these embryos because many donkey populations around the world are declining. It may sound counterintuitive that domesticated animals may need preservation, but out of 28 European donkey breeds, 20 are endangered and seven are in critical status. It is partly because of the inbreeding that happened over the course of many years and partly because in today’s Western world donkeys aren’t really used anymore.
“That's the reason why some breeds begin to disappear because humans were not really interested in having that specific breed anymore,” Gambini says. Nonetheless, in Africa, India and Latin America millions of rural families still rely on these hardy creatures for agriculture and transportation. And the only two wild donkey species—Equus africanus in Africa and Equus hemionus in Asia—are also dwindling, due to losing their habitats to human activities, diseases and slow reproduction rates. Gambini’s team wanted to create a way to preserve the animals for the future. “Donkeys are more endangered than people realize,” he says.
There’s much more to donkeys' trouble though. For the past 20 or so years, they have been facing a huge existential threat due to their hide gelatin, a compound derived from their skins by soaking and stewing. In Chinese traditional medicine, the compound, called ejiao, is believed to have a medicinal value, so it’s used in skin creams, added to food and taken in capsules. Centuries ago, ejiao was a very expensive luxury product available only for the emperor and his household. That changed in the 1990s when the Chinese economy boomed, and many people were suddenly able to afford it. “It went from a very elite product to a very popular product,” says Janneke Merkx, a campaign manager at The Donkey Sanctuary, a United Kingdom-based nonprofit organization that keeps tabs on the animals’ welfare worldwide. “It is a status symbol for gift giving.”
Having evolved in the harsh and arid mountainous terrains where food and water were scarce, donkeys are extremely adaptable and hardy. But the Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement.
The Chinese donkey population was quickly decimated. Unlike many other farm animals, donkeys are finicky breeders. When stressed and unhappy, they don’t procreate, so growing them in large industrial settings isn’t possible. “Donkeys are notoriously slow breeders and really very difficult to farm,” says Merkx. “They are not the same as other livestock like sheep and pigs and cattle.” Within years the, the donkey numbers in China dropped precipitously. “China used to have the largest donkey population in the world in the 1990s. They had 11 million donkeys, and it's now down to less than 3 million, and they just can't keep up with the demand.”
To keep the ejiao conveyor going, some producers turned to the illegal wildlife trade. Poachers began to steal and slaughter donkeys from rural villages in Africa. The Donkey Sanctuary documented cases in which an entire village had their animals disappear overnight, finding them killed and skinned outside their settlement. Exactly how many creatures were lost to the skin trade to-date isn’t possible to calculate, says Faith Burden, the Donkey Sanctuary’s director of equine operations. Traditionally a poor people’s beast of burden, donkey counts are hard to keep track of. “When an animal doesn't produce meat, milk or eggs or whatever edible product, they're often less likely to be acknowledged in a government population census,” Burden says. “So reliable statistics are hard to come by.” The nonprofit estimates that about 4.8 million are slaughtered annually.
During their six to seven thousand years of domestication, donkeys rarely got the full appreciation for their services. They are often compared to horses, which doesn’t do them justice. They’re entirely different animals, Burden says. Built for speed, horses respond to predators and other dangers by running as fast as they can. Donkeys, which originate from the rocky, mountainous regions of Africa where running is dangerous, react to threats by freezing and assessing the situation for the best response. “Those so-called stubborn donkeys that won’t move as you want, they are actually thinking ‘what’s the best approach,’” Burden says. They may even choose to fight the predators rather than flee, she adds. “In some parts of the world, people use them as guard animals against things like coyotes and wolves.”
Scientists believe that domestic donkeys take their origin from Equus africanus or African wild ass, originally roaming where Kenya, Ethiopia and Eritrea are today. Having evolved in the harsh and arid mountainous terrains where food and water were scarce, they are extremely adaptable and hardy. Research finds that they can go without water for 72 hours and then drink their fill without any negative consequences. Their big jaws let them chew tough desert shrubs, which horses can’t exist on. Their large ears help dissipate heat. Their little upright hooves are a perfect fit for the uneven rocky or other dangerous grounds. Accustomed to the mountain desert climate with hot days and cold nights, they don’t mind temperature flux.
“The donkey is the most supremely adapted animal to deal with hostile conditions,” Burden says. “They can survive on much lower nutritional quality food than a cow, sheep or horse. That’s why communities living in some of the most inhospitable places will often have donkeys with them.” And that’s why losing a donkey to an illegal skin trade can devastate a family in places like Eritrea. Suddenly everything from water to firewood to produce must be carried by family members—and often women.

Workers unloading donkeys at the Shinyanga slaughterhouse in Tanzania. Fearing a future in which donkeys go extinct, scientists have found ways to cryopreserve a donkey embryo in liquid nitrogen.
TAHUCHA
One can imagine a time when worldwide donkey populations may dwindle to the point that they would need to be restored. That includes their genetic variability too. That’s where the frozen embryos may come in handy. We may be able to use them to increase the genetic variability of donkeys, which will be especially important if they get closer to extinction, Gambini says. His team had already created frozen embryos for horses and zebras, an idea similar to a seed bank. “We call this concept the Frozen Zoo.”
Creating donkey embryos proved much harder than those of zebras and horses. To improve chances of fertilization, Gambini used the intracytoplasmic sperm injection or ICSI, in which he employed a tiny needle called a micropipette to inject a donkey sperm into an egg. That was a step above the traditional IVF method, in which the egg and a sperm are left floating in a test tube together. The injection took, but during the incubating week, one after the other, the embryos stopped dividing. Finally, on day seven, Gambini finally spotted the exact sight he was hoping to see. One of the embryos developed into a burgeoning ball of cells.
“That stage is called a blastocyst,” Gambini says. The clump of cells had a lot of fluids mixed within them, which indicated that they were finally developing into a viable embryo. “When we see a blastocyst, we know we can transfer that into a female.” He was so excited he immediately called all his collaborators to tell them the good news, which they later published in the journal of Theriogenology.
The one and only embryo to reach that stage, the blastocyst was cryopreserved in liquid nitrogen. The team is waiting for the next breeding season to see if a female donkey may carry it to term and give birth to a healthy foal. Gambini’s team is hoping to polish the process and create more embryos. “It’s our weapon in the conservation ass-enal,” he says.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Too much of this ingredient leads to autoimmune diseases, new research shows. Here's how to cut back.
Scientists are looking at how salt affects our cells, and they're finding more reasons to avoid htoo much of it.
For more than a century, doctors have warned that too much salt in your diet can lead to high blood pressure, heart disease and stroke - and many of the reasons for these effects are well known. But recently scientists have been looking deeper, into the cellular level, and they are finding additional reasons to minimize sodium intake; it is bad for immune cells, creating patterns of gene expression and activity seen in a variety of autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, and type-1 diabetes.
Salt is a major part of the ocean from which life evolved on this planet. We carry that legacy in our blood, which tastes salty. It is an important element for conducting electrical signals along nerves and balancing water and metabolites transported throughout our bodies. We need to consume about 500 milligrams of salt each day to maintain these functions, more with exercise and heavy sweating as that is a major way the body loses salt. The problem is that most Americans eating a modern western diet consume about 3400 milligrams, 1.5 teaspoons per day.
Evidence has been accumulating over the last few years that elevated levels of sodium can be harmful to at least some types of immune cells. The first signal came in monocytes, which are immune cells that travel to various tissues in the body, where some of them turn into macrophages, a subset of white blood cells that can directly kill microorganisms and make chemical signals that bring other types of immune cells into play.
Two years ago, Dominik N. Müller from the Max-Delbrueck-Center in Berlin, Germany and Markus Kleinewietfeld, an immunologist at Hasselt University in Belgium, ran a study where they fed people pizza and then measured their immune cell function. “We saw that in any monocytes, metabolic function was down, even after a single salty meal,” Kleinewietfeld says. It seemed to be the cellular equivalent of the sluggish feeling we get after eating too much. The cells were able to recover but more research is needed to answer questions about what dose of sodium causes impairment, how long the damage lasts, and whether there is a cumulative effect of salt toxicity.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations.
The latest series of experiments focused on a type of T cell called T regulatory cells, or Tregs. Most T cells release inflammatory mediators to fight pathogens and, once that job is done, Tregs come along to calm down their hyperactive brethren. Failure to do so can result in continued inflammation and possibly autoimmune diseases.
In the lab, Kleinewietfeld and his large team of international collaborators saw that high levels of sodium had a huge effect on Tregs, upregulating 1250 genes and downregulating an additional 1380 genes so that they looked similar to patterns of gene expression seen in autoimmune diseases.
Digging deeper, they found that sodium affected mitochondria, the tiny organelles inside of cells that produce much of its energy. The sodium was interfering with how the mitochondria use oxygen, which resulted in increased levels of an unstable form of oxygen that can damage cell function. The researchers injected those damaged Tregs into mice and found that they impaired the animals' immune function, allowing the inflammation to continue rather than shutting it down.
That finding dovetailed nicely with a 2019 paper in Nature from Navdeep Chandel's lab at Northwestern University, which showed in mice that inhibiting the mitochondrial use of oxygen reduced the ability of Tregs to regulate other T cells. “Mitochondria were controlling directly the immunosuppressive program, they were this master regulator tuning the right amount of genes to give you proper immunosuppression,” Chandel said. “And if you lose that function, then you get autoimmunity.”
Kleinewietfeld's team studied the Treg cells of humans and found that sodium can similarly decrease mitochondrial use of oxygen and immunosuppressive activity. “I would have never predicted that myself,” Chandel says, but now researchers can look at the mitochondria of patients with autoimmune disease and see if their gene expression also changes under high salt conditions. He sees the link between the patterns of gene expression in Tregs generated by high salt exposure and those patterns seen in autoimmune diseases, but he is cautious about claiming a causal effect.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations. He says a high salt diet could also have an indirect effect on immune function through the way it affects the gut microbiome and the molecules made by microbes when they break down food. But the research results are too preliminary to say that for sure, much less parse out the role of salt compared with other possible factors. “It is still an exciting journey to try to understand this field,” he says.
Additionally, it is difficult to say precisely how this research in animals and human cell cultures will translate into a whole human body. Individual differences in genetics can affect how the body absorbs, transports, and gets rid of sodium, such that some people are more sensitive to salt than are others.
So how should people apply these research findings to daily life?
Salt is obvious when we sprinkle it on at the table or eat tasty things like potato chips, but we may be unaware of sodium hidden in packaged foods. That's because salt is an easy and cheap way to boost the flavor of foods. And if we do read the labeled salt content on a package, we focus on the number for a single serving, but then eat more than that.
Last September, the U.S. Food and Drug Administration (FDA) began a process to update labels on the content of food, including what is meant by the word “healthy” and how food manufacturers can use the term. Many in the food industry are resisting those proposed changes.
Chandel cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker.
Until labels are updated, it would be prudent to try to reduce sodium intake by cutting down on packaged foods while making your own food at home, where you know just how much salt has been added. The Mayo Clinic offers guidance on how to become more aware of the sodium in your diet and eat less of it.
Chandel thinks many people will struggle with minimizing salt in their diets. It’s similar to the challenge of eating less sugar, in that the body craves both, and it is difficult to fight that. He cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker. “Dietary antioxidants have failed in just about every clinical trial, yet the public continues to take them,” Chandel says. But he is optimistic that research will lead us to a better understanding of how Tregs function, and uncover new targets for treating autoimmune diseases.

