This Boy Struggled to Walk Before Gene Therapy. Now, Such Treatments Are Poised to Explode.

Conner Curran, now 10 years old, can walk more than two miles after gene therapy treatment for his Duchenne's muscular dystrophy.
Conner Curran was diagnosed with Duchenne's muscular dystrophy in 2015 when he was four years old. It's the most severe form of the genetic disease, with a nearly inevitable progression toward total paralysis. Many Duchenne's patients die in their teens; the average lifespan is 26.
But Conner, who is now 10, has experienced some astonishing improvements in recent years. He can now walk for more than two miles at a time – an impossible journey when he was younger.
In 2018, Conner became the very first patient to receive gene therapy specific to treating Duchenne's. In the initial clinical trial of nine children, nearly 80 percent reacted positively to the treatment). A larger-scale stage 3 clinical trial is currently underway, with initial results expected next year.
Gene therapy involves altering the genes in an individual's cells to stop or treat a disease. Such a procedure may be performed by adding new gene material to existing cells, or editing the defective genes to improve their functionality.
That the medical world is on the cusp of a successful treatment for a crippling and deadly disease is the culmination of more than 35 years of work by Dr. Jude Samulski, a professor of pharmacology at the University of North Carolina School of Medicine in Chapel Hill. More recently, he's become a leading gene therapy entrepreneur.
But Samulski likens this breakthrough to the frustrations of solving a Rubik's cube. "Just because one side is now all the color yellow does not mean that it is completely aligned," he says.
Although Conner's life and future have dramatically improved, he's not cured. The gene therapy tamed but did not extinguish his disorder: Conner is now suffering from the equivalent of Becker's muscular dystrophy, a milder form of the disease with symptoms that appear later in life and progress more slowly. Moreover, the loss of muscle cells Conner suffered prior to the treatment is permanent.
"It will take more time and more innovations," Samulski says of finding an even more effective gene therapy for muscular dystrophy.
Conner's family is still overjoyed with the results. "Jude's grit and determination gave Conner a chance at a new life, one that was not in his cards before gene therapy," says his mother Jessica Curran. She adds that "Conner is more confident than before and enjoys life, even though he has limitations, if compared to his brothers or peers."

Conner Curran holding a football post gene therapy treatment.
Courtesy of the Curran family
For now, the use of gene therapy as a treatment for diseases and disorders remains relatively isolated. On paper at least, progress appears glacially slow. In 2018, there were four FDA-approved gene therapies (excluding those reliant on bone marrow/stem cell transplants or implants). Today, there are 10. One therapy is solely for the cosmetic purpose of reducing facial lines and folds.
Nevertheless, experts in the space believe gene therapy is poised to expand dramatically.
"Certainly in the next three to five years you will see dozens of gene therapies and cell therapies be approved," says Dr. Pavan Cheruvu, who is CEO of Sio Gene Therapies in New York. The company is developing treatments for Parkinson's disease and Tay-Sachs, among other diseases.
Cheruvu's conclusion is supported by NEWDIGS, a think tank at the Massachusetts Institute of Technology that keeps tabs on gene therapy developments. NEWDIGS predicts there will be at least 60 gene therapies approved for use in the U.S. by the end of the decade. That number could be closer to 100 if Chinese researchers and biotech ventures decide the American market is a good fit for the therapies they develop.
"We are watching something of a conditional evolution, like a dot-com, or cellphones that were sizes of shoeboxes that have now matured to the size of wafers. Our space will follow along very similarly."
Dr. Carsten Brunn, a chemist by training and CEO of Selecta Biosciences outside of Boston, is developing ways to reduce the immune responses in patients who receive gene therapy. He observes that there are more than 300 therapies in development and thousands of clinical trials underway. "It's definitely an exciting time in the field," he says.
That's a far cry from the environment of little more than a decade ago. Research and investment in gene therapy had been brought low for years after the death of teenager Jesse Gelsinger in 1999 while he had been enrolled in a clinical trial to treat a liver disease. Gene therapy was a completely novel concept back then, and his death created existential questions about whether it was a proper pathway to pursue. Cheruvu, a cardiologist, calls the years after Gelsinger's death an "ice age" for gene therapy.
However, those dark years eventually yielded to a thaw. And while there have been some recent stumbles, they are considered part of the trial-and-error that has often accompanied medical research as opposed to an ominous "stop" sign.
The deaths of three patients last year receiving gene therapy for myotubular myopathy – a degenerative disease that causes severe muscle weakness – promptly ended the clinical trial in which they were enrolled. However, the incident caused few ripples beyond that. Researchers linked the deaths to dosage sizes that caused liver toxicity, as opposed to the gene therapy itself being an automatic death sentence; younger patients who received lower doses due to a less advanced disease state experienced improvements.
The gene sequencing and editing that helped create vaccines for COVID-19 in record time also bolstered the argument for more investment in research and development. Cheruvu notes that the field has usually been the domain of investors with significant expertise in the field; these days, more money is flowing in from generalists.
The Challenges Ahead
What will be the next step in gene therapy's evolution? Many of Samulski's earliest innovations came in the laboratory, for example. Then that led to him forming a company called AskBio in collaboration with the Muscular Dystrophy Association. AskBio sold its gene therapy to Pfizer five years ago to assure that enough could be manufactured for stage 3 clinical trials and eventually reach the market.
Cheruvu suggests that many future gene therapy innovations will be the result of what he calls "congruent innovation." That means publicly funded laboratories and privately funded companies might develop treatments separately or in collaboration. Or, university scientists may depend on private ventures to solve one of gene therapy's most vexing issues: producing enough finished material to test and treat on a large scale. "Manufacturing is a real bottleneck right now," Brunn says.
The alternative is referred to in the sector as the "valley of death": a lab has found a promising treatment, but is not far enough along in development to submit an investigational new drug application with the FDA. The promise withers away as a result. But the new abundance of venture capital for gene therapy has made this scenario less of an issue for private firms, some of which have received hundreds of millions of dollars in funding.
There are also numerous clinical challenges. Many gene therapies use what are known as adeno-associated virus vectors (AAVs) to deliver treatments. They are hollowed-out husks of viruses that can cause a variety of mostly mild maladies ranging from colds to pink eye. They are modified to deliver the genetic material used in the therapy. Most of these vectors trigger an antibody reaction that limits treatments to a single does or a handful of smaller ones. That can limit the potential progress for patients – an issue referred to as treatment "durability."
Although vectors from animals such as horses trigger far less of an antibody reaction in patients -- and there has been significant work done on using artificial vectors -- both are likely years away from being used on a large scale. "For the foreseeable future, AAV is the delivery system of choice," Brunn says.
Also, there will likely be demand for concurrent gene therapies that can lead to a complete cure – not only halting the progress of Duchenne's in kids like Conner Curran, but regenerating their lost muscle cells, perhaps through some form of stem cell therapy or another treatment that has yet to be devised.
Nevertheless, Samulski believes demand for imperfect treatments will be high – particularly with a disease such as muscular dystrophy, where many patients are mere months from spending the remainder of their lives in wheelchairs. But Samulski believes those therapies will also inevitably evolve into something far more effective.
"We are watching something of a conditional evolution, like a dot-com, or cellphones that were sizes of shoeboxes that have now matured to the size of wafers," he says. "Our space will follow along very similarly."
Jessica Curran will remain forever grateful for what her son has received: "Jude gave us new hope. He gave us something that is priceless – a chance to watch Conner grow up and live out his own dreams."
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
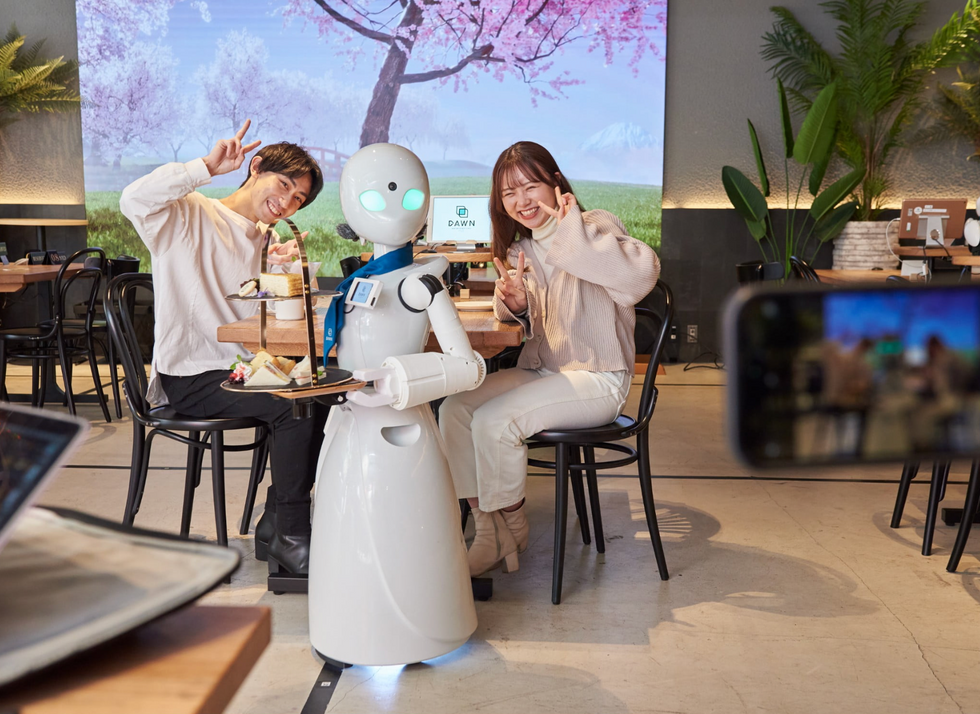
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
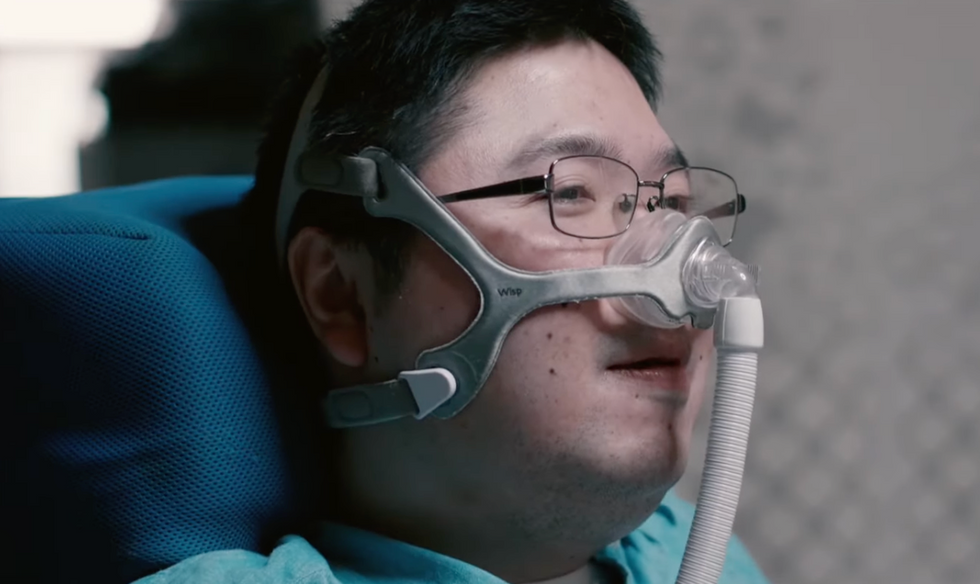
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

