An At-Home Contagiousness Test for COVID-19 Already Exists. Why Can’t We Use It?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

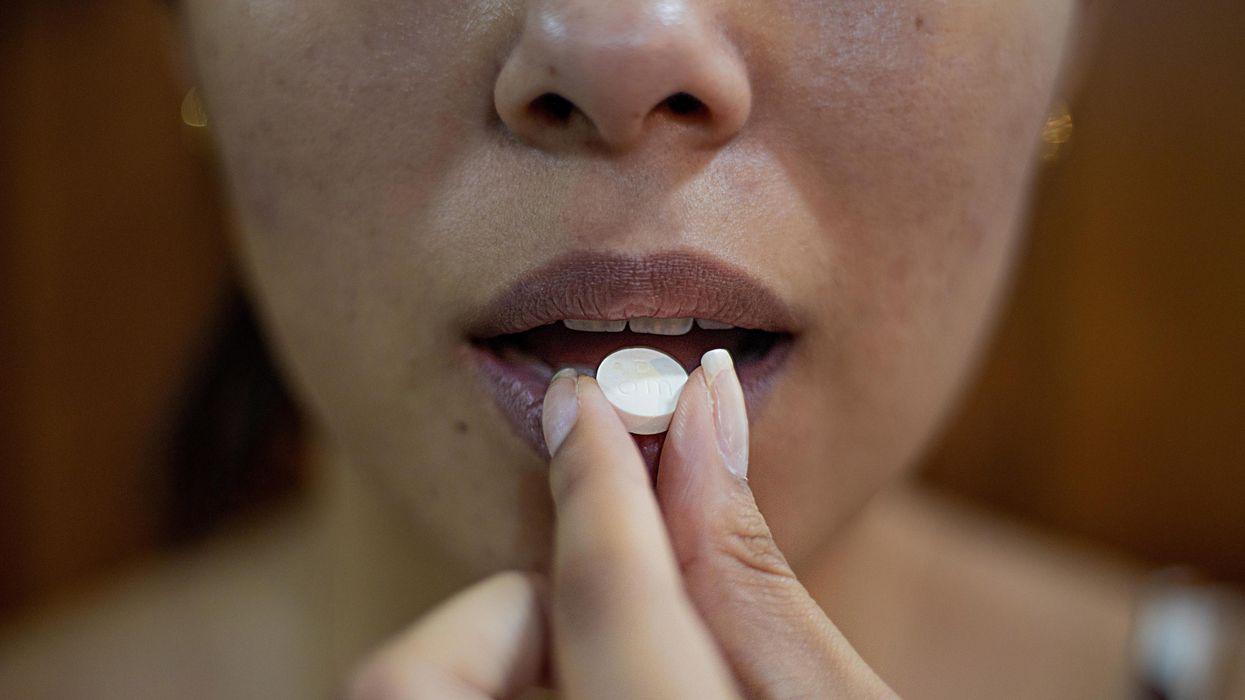
Bobby Brooke Herrera, the co-founder and CEO of e25Bio, demonstrates the company's rapid paper-strip test for detecting the coronavirus.
You're lying in bed late at night, the foggy swirl of the pandemic's 8th month just beginning to fall behind you, when you detect a slight tickle at the back of your throat.
"If half of people choose to use these tests every other day, then we can stop transmission faster than a vaccine can."
Suddenly fully awake, a jolt of panicked electricity races through your body. Has COVID-19 come for you? In the U.S., answering this simple question is incredibly difficult.
Now, you might have to wait for hours in line in your car to get a test for $100, only to find out your result 10-14 days later -- much too late to matter in stopping an outbreak. Due to such obstacles, a recent report in JAMA Internal Medicine estimated that 9 out of 10 infections in the U.S. are being missed.
But what if you could use a paper strip in the privacy of your own home, like a pregnancy test, and find out if you are contagious in real time?
e25 Bio, a small company in Cambridge, Mass., has already created such a test and it has been sitting on a lab bench, inaccessible, since April. It is an antigen test, which looks for proteins on the outside of a virus, and can deliver results in about 15 minutes. Also like an over-the-counter pregnancy test, e25 envisions its paper strips as a public health screening tool, rather than a definitive diagnostic test. People who see a positive result would be encouraged to then seek out a physician-administered, gold-standard diagnostic test: the more sensitive PCR.
Typically, hospitals and other health facilities rely on PCR tests to diagnose viruses. This test can detect small traces of genetic material that a virus leaves behind in the human body, which tells a clinician that the patient is either actively infected with or recently cleared that virus. PCR is quite sensitive, meaning that it is able to detect the presence of a virus' genetic material very accurately.
But although PCR is the gold-standard for diagnostics, it's also the most labor-intensive way to test for a virus and takes a relatively long time to produce results. That's not a good match for stopping super-spreader events during an unchecked pandemic. PCR is also not great at identifying the infected people when they are most at risk of potentially transmitting the virus to others.
That's because the viral threshold at which PCR can detect a positive result is so low, that it's actually too sensitive for the purposes of telling whether someone is contagious.
"The majority of time someone is PCR positive, those [genetic] remnants do not indicate transmissible virus," epidemiologist Michael Mina recently Tweeted. "They indicate remnants of a recently cleared infection."
To stop the chain of transmission for COVID-19, he says, "We need a more accurate test than PCR, that turns positive when someone is able to transmit."
In other words, we need a test that is better at detecting whether a person is contagious, as opposed to whether a small amount of virus can be detected in their nose or saliva. This kind of test is especially critical given the research showing that asymptomatic and pre-symptomatic people have high viral loads and are spreading the virus undetected.
The critical question for contagiousness testing, then, is how big a dose of SARS-CoV-2, the virus that causes COVID, does it take to infect most people? Researchers are still actively trying to answer this. As Angela Rasmussen, a coronavirus expert at Columbia University, told STAT: "We don't know the amount that is required to cause an infection, but it seems that it's probably not a really, really small amount, like measles."
Amesh Adalja, an infectious disease physician and a senior scholar at the Johns Hopkins University Center for Health Security, told LeapsMag: "It's still unclear what viral load is associated with contagiousness but it is biologically plausible that higher viral loads, in general, are associated with more efficient transmission especially in symptomatic individuals. In those without symptoms, however, the same relationship may not hold and this may be one of the reasons young children, despite their high viral loads, are not driving outbreaks."
"Antigen tests work best when there's high viral loads. They're catching people who are super spreaders."
Mina and colleagues estimate that widespread use of weekly cheap, rapid tests that are 100 times less sensitive than PCR tests would prevent outbreaks -- as long as the people who are positive self-isolate.
So why can't we buy e25Bio's test at a drugstore right now? Ironically, it's barred for the very reason that it's useful in the first place: Because it is not sensitive enough to satisfy the U.S. Food and Drug Administration, according to the company.
"We're ready to go," says Carlos-Henri Ferré, senior associate of operations and communications at e25. "We've applied to FDA, and now it's in their hands."
The problem, he said, is that the FDA is evaluating applications for antigen tests based on criteria for assessing diagnostics, like PCR, even when the tests serve a different purpose -- as a screening tool.
"Antigen tests work best when there's high viral loads," Ferré says. "They're catching people who are super spreaders, that are capable of continuing the spread of disease … FDA criteria is for diagnostics and not this."
FDA released guidance on July 29th -- 140 days into the pandemic -- recommending that at-home tests should perform with at least 80 percent sensitivity if ordered by prescription, and at least 90 percent sensitivity if purchased over the counter. "The danger of a false negative result is that it can contribute to the spread of COVID-19," according to an FDA spokesperson. "However, oversight of a health care professional who reviews the results, in combination with the patient's symptoms and uses their clinical judgment to recommend additional testing, if needed, among other things, can help mitigate some risks."
Crucially, the 90 percent sensitivity recommendation is judged upon comparison to PCR tests, meaning that if a PCR test is able to detect virus in 100 samples, the at-home antigen test would need to detect virus in at least 90 of those samples. Since antigen tests only detect high viral loads, frustrated critics like Mina say that such guidance is "unreasonable."
"The FDA at this moment is not understanding the true potential for wide-scale frequent testing. In some ways this is not their fault," Mina told LeapsMag. "The FDA does not have any remit to evaluate tests that fall outside of medical diagnostic testing. The proposal I have put forth is not about diagnostic testing (leave that for symptomatic cases reporting to their physician and getting PCR tests)....Daily rapid tests are not about diagnosing people and they are not about public health surveillance and they are not about passports to go to school, out to dinner or into the office. They are about reducing population-level transmission given a similar approach as vaccines."
A reasonable standard, he added, would be to follow the World Health Organization's Target Product Profiles, which are documents to help developers build desirable and minimally acceptable testing products. "A decent limit," Mina says, "is a 70% or 80% sensitivity (if they truly require sensitivity as a metric) to detect virus at Ct values less than 25. This coincides with detection of the most transmissible people, which is important."
(A Ct value is a type of measurement that corresponds inversely to the amount of viral load in a given sample. Researchers have found that Ct values of 13-17 indicate high viral load, whereas Ct values greater than 34 indicate a lack of infectious virus.)
"We believe this should be an at-home test, but [if FDA approval comes through] the first rollout is to do this in laboratories, hospitals, and clinics."
"We believe that population screening devices have an immediate place and use in helping beat the virus," says Ferré. "You can have a significant impact even with a test at 60% sensitivity if you are testing frequently."
When presented with criticism of its recommendations, the FDA indicated that it will not automatically deny any at-home test that fails to meet the 90 percent sensitivity guidance.
"FDA is always open to alternative proposals from developers, including strategies for serial testing with less sensitive tests," a spokesperson wrote in a statement. "For example, it is possible that overall sensitivity of the strategy could be considered cumulatively rather than based on one-time testing….In the case of a manufacturer with an at-home test that can only detect people with COVID-19 when they have a high viral load, we encourage them to talk with us so we can better understand their test, how they propose to use it, and the validation data they have collected to support that use."
However, the FDA's actions so far conflict with its stated openness. e25 ended up adding a step to the protocol in order to better meet FDA standards for sensitivity, but that extra step—sending samples to a laboratory for results—will undercut the test's ability to work as an at-home screening tool.
"We believe this should be an at-home test, but [if FDA approval comes through] the first rollout is to do this in laboratories, hospitals, and clinics," Ferré says.
According to the FDA, no test developers have approached them with a request for an emergency use authorization that proposes an alternate testing paradigm, such as serial testing, to mitigate test sensitivity below 80 percent.
From a scientific perspective, antigen tests like e25Bio's are not the only horse in the race for a simple rapid test with potential for at-home use. CRISPR technology has long been touted as fertile ground for diagnostics, and in an eerily prescient interview with LeapsMag in November, CRISPR pioneer Feng Zhang spoke of its potential application as an at-home diagnostic for an infectious disease specifically.
"I think in the long run it will be great to see this for, say, at-home disease testing, for influenza and other sorts of important public health [concerns]," he said in the fall. "To be able to get a readout at home, people can potentially quarantine themselves rather than traveling to a hospital and then carrying the risk of spreading that disease to other people as they get to the clinic."
Zhang's company Sherlock Biosciences is now working on scaled-up manufacturing of a test to detect SARS CoV-2. Mammoth Biosciences, which secured funding from the National Institutes of Health's Rapid Acceleration of Diagnostics program, is also working on a CRISPR diagnostic for SARS CoV-2. Both would check the box for rapid testing, but so far not for at-home testing, as they would also require laboratory infrastructure to provide results.
If any at-home tests can clear the regulatory hurdles, they would also need to be manufactured on a large scale and be cheap enough to entice people to actually use them. In the world of at-home diagnostics, pregnancy tests have become the sole mainstream victor because they're simple to use, small to carry, easy to interpret, and costs about seven or eight dollars at any ubiquitous store, like Target or Walmart. By comparison, the at-home COVID collection tests that don't even offer diagnostics—you send away your sample to an external lab—all cost over $100 to take just one time.
For the time being, the only available diagnostics for COVID require a lab or an expensive dedicated machine to process. This disconnect could prolong the world's worst health crisis in a century.
"Daily rapid tests have enormous potential to sever transmission chains and create herd effects similar to herd immunity," Mina says. "We all recognize that vaccines and infections can result in herd immunity when something around half of people are no longer susceptible.
"The same thing exists with these tests. These are the intervention to stop the virus. If half of people choose to use these tests every other day, then we can stop transmission faster than a vaccine can. The technology exists, the theory and mathematics back it up, the epidemiology is sound. There is no reason we are not approaching this as strongly as we would be approaching vaccines."
--Additional reporting by Julia Sklar
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
CandyCodes could provide sweet justice against fake pills
A bioengineer at the University of California, Riverside, may have found a way to prevent counterfeit medications: pill coatings inspired by the sprinkles on baked goods and candies.
When we swallow a pill, we hope it will work without side effects. Few of us know to worry about a growing issue facing the pharmaceutical industry: counterfeit medications. These pills, patches, and other medical products might look just like the real thing. But they’re often stuffed with fillers that dilute the medication’s potency or they’re simply substituted for lookalikes that contain none of the prescribed medication at all.
Now, bioengineer William Grover at the University of California, Riverside, may have a solution. Inspired by the tiny, multi-colored sprinkles called nonpareils that decorate baked goods and candies, Grover created CandyCodes pill coatings to prevent counterfeits.
The idea was borne out of pandemic boredom. Confined to his home, Grover was struck by the patterns of nonpareils he saw on candies, and found himself counting the number of little balls on each one. “It’s random, how they’re applied,” he says. “I wondered if it ever repeats itself or if each of these candies is unique in the entire world.” He suspected the latter, and some quick math proved his hypothesis: Given dozens of nonpareils per candy in a handful of different colors, it’s highly unlikely that the sprinklings on any two candies would be identical.
He quickly realized his finding could have practical applications: pills or capsules could be coated with similar “sprinkles,” with the manufacturer photographing each pill or capsule before selling its products. Consumers looking to weed out fakes could potentially take a photo with their cell phones and go online to compare images of their own pills to the manufacturer’s database, with the help of an algorithm that would determine their authenticity. Or, a computer could generate another type of unique identifier, such as a text-based code, tracking to the color and location of the sprinkles. This would allow for a speedier validation than a photo-based comparison, Grover says. “It could be done very quickly, in a fraction of a second.”
Researchers and manufacturers have already developed some anti-counterfeit tools, including built-in identifiers like edible papers with scannable QR codes. But such methods, while functional, can be costly to implement, Grover says.
It wouldn’t be paranoid to take such precautions. Counterfeits are a growing problem, according to Young Kim, a biomedical engineer at Purdue University who was not involved in the CandyCodes study. “There are approximately 40,000 online pharmacies that one can access via the Internet,” he says. “Only three to four percent of them are operated legally.” Purchases from online pharmacies rose dramatically during the pandemic, and Kim expects a boom in counterfeit medical products alongside it.
The FDA warns that U.S. consumers can be exposed to counterfeits through online purchases, in particular. The problem is magnified in low- to middle-income nations, where one in 10 medical products are counterfeit, according to a World Health Organization estimate. Cost doesn’t seem to be a factor, either; antimalarials and antibiotics are most often reported as counterfeits or fakes, and generic medications are swapped as often as brand-name drugs, according to the same WHO report.
Counterfeits weren’t tracked globally until 2013; since then, there have been 1,500 reports to the WHO, with actual incidences of counterfeiting likely much higher. Fake medicines have been estimated to result in costs of $200 billion each year, and are blamed for more than 72,000 pneumonia- and 116,000 malaria-related deaths.
Researchers and manufacturers have already developed some anti-counterfeit tools, including built-in identifiers like edible papers with scannable QR codes or barcodes that are stamped onto or otherwise incorporated into pills and other medical products. But such methods, while functional, can be costly to implement, Grover says.

CandyCodes could provide unique identifiers for at least 41 million pills for every person on the planet.
William Grover
“Putting universal codes on each pill and each dosage is attractive,” he says. “The challenge is, how can we do it in a way that requires as little modification to the existing manufacturing process as possible? That's where I hope CandyCodes have an edge. It's not zero modification, but I hope it is as minor a modification of the manufacturing process as possible.”
Kim calls the concept “a clever idea to introduce entropy for high-level security” even if it may not be as close to market as other emerging technologies, including some edible watermarks he’s helped develop. He points out that CandyCodes still needs to be tested for reproducibility and readability.
The possibilities are already intriguing, though. Grover’s recent research, published in Scientific Reports, predicts that unique codes could be used for at least 41 million pills for every person on the planet.
Sadly, CandyCodes’ multicolored bits probably won’t taste like candy. They must be made of non-caloric ingredients to meet the international regulatory standards that govern food dyes and colorants. But Grover hopes CandyCodes represent a simple, accessible solution to a heart-wrenching issue. “This feels like trying to track down and go after bad guys,” he says. “Someone who would pass off a medicine intended for a child or a sick person and pass it off as something effective, I can't imagine anything much more evil than that. It's fun and, and a little fulfilling to try to develop technologies that chip away at that.”
Waste smothering our oceans is worth billions – here’s what we can do with all that sh$t
In 2015, human poop was valued at $9.5 billion per year, which today would be $11.5 billion. The Ocean Sewage Alliance is uniting experts from key sectors to change how we handle our sewage and, in the process, create all sorts of economic benefits.
There’s hardly a person out there who hasn’t heard of the Great Pacific Garbage Patch. That type of pollution is impossible to miss. It stares you in the face from pictures and videos of sea turtles with drinking straws up their noses and acres of plastic swirling in the sea.
It demands you to solve the problem—and it works. The campaign to raise awareness about plastic pollution in the oceans has resulted in new policies, including bans on microplastics in personal care products, technology to clean up the plastic, and even new plastic-like materials that are better for the environment.
But there’s a different type of pollution smothering the ocean as you read this. Unfortunately, this one is almost invisible, but no less damaging. In fact, it’s even more serious than plastic and most people have no idea it even exists. It is literally under our noses, destroying our oceans, lakes, and rivers – and yet we are missing it completely while contributing to it daily. In fact, we exacerbate it multiple times a day—every time we use the bathroom.
It is the way we do our sewage.
Most of us don’t think much about what happens after we flush the toilet. Most of us probably assume that the substances we flush go “somewhere” and are dealt with safely. But we typically don’t think about it beyond that.
Most of us also probably don’t think about what’s in the ocean or lakes we swim in. Since others are swimming, jumping in is just fine. But our waterways are far from clean. In fact, at times they are incredibly filthy. In the US, we are dumping 1.2 trillion of gallons of untreated sewage into the environment every year. Just New York City alone discharges 27 billion gallons into the Hudson River basin annually.
How does this happen? Part of it is the unfortunate side effect of our sewage system design that dates back to over a century ago when cities were smaller and fewer people were living so close together.
Back then, engineers designed the so-called “combine sewer overflow systems,” or CSOs, in which the storm water pipes are connected to the sanitary sewer pipes. In normal conditions, the sewage effluent from homes flows to the treatment plants where it gets cleaned and released into the waterways. But when it rains, the pipe system becomes so overwhelmed with water that the treatment plant can’t process it fast enough. So the treatment plant has to release the excess water through its discharge pipes—directly, without treatment, into streams, rivers and the ocean.
The 1.2 trillion gallons of CSO releases isn’t even the full picture. There are also discharges from poorly maintained septic systems, cesspools and busted pipes of the aging wastewater infrastructure. The state of Hawaii alone has 88,000 cesspools that need replacing and are currently leaking 53 million gallons of raw sewage daily into their coastal waters. You may think twice about swimming on your Hawaii vacations.
Overall, the US is facing a $271 billion backlog in wastewater infrastructure projects to update these aging systems. Across the Western world, countries are facing similar challenges with their aging sewage systems, especially the UK and European Union.
That’s not to say that other parts of the planet are in better shape. Out of the 7+ billion people populating our earth, 4.2 billion don’t have access to safe sanitation. Included in this insane number are roughly 2 billion people who have no toilet at all. Whether washed by rains or dumped directly into the waterways, a lot of this sludge pollutes the environment, the drinking water, and ultimately the ocean.

Pipes pour water onto a rocky shore in Jakarta, Indonesia.
Tom Fisk
What complicates this from an ocean health perspective is that it’s not just poop and pee that gets dumped into nearby waterways. It is all the things we put in and on our bodies and flush down our drains. That vicious mix of chemicals includes caffeine, antibiotics, antidepressants, painkillers, hormones, microplastics, cocaine, cooking oils, paint thinners, and PFAS—the forever chemicals present in everything from breathable clothing to fire retardant fabrics of our living room couches. Recent reports have found all of the above substances in fish—and then some.
Why do we allow so much untreated sewage spill into the sea? Frankly speaking, for decades scientists and engineers thought that the ocean could handle it. The mantra back then was “dilution is the solution to pollution,” which might’ve worked when there were much fewer people living on earth—but not now. Today science is telling us that this old approach doesn’t hold. That marine habitats are much more sensitive than we had expected and can’t handle the amount of wastewater we are discharging into them.
The excess nitrogen and phosphorus that the sewage (and agricultural runoff) dumps into the water causes harmful algal blooms, more commonly known as red or brown tides. The water column is overtaken by tiny algae that sucks up all the oxygen from the water, creating dead zones like the big fish kills in the Gulf of Mexico. These algae also cause public health issues by releasing gases toxic to people and animals, including dementia, neurological damage, and respiratory illness. Marshes and mangroves end up with weakened root systems and start dying off. In a wastewater modeling study I published last year, we found that 31 percent of salt marshes globally were heavily polluted with human sewage. Coral reefs get riddled with disease and overgrown by seaweed.
We could convert sewage into high-value goods. It can be used to generate electricity, fertilizer, and drinking water. The technologies not only exist but are getting better and more efficient all the time.
Moreover, by way of our sewage, we managed to transmit a human pathogen—Serratia marcescens, which causes urinary, respiratory and other infections in people—to corals! Recent reports from the Florida Keys are showing white pox disease popping up in elk horn corals caused by S.marcescens, which somehow managed to jump species. Many recent studies have documented just how common this type of pollution is across the globe.
Yet, there is some good news in that abysmal sewage flow. Just like with plastic pollution, realizing that there’s a problem is the first step, so awareness is key. That’s exactly why I co-founded Ocean Sewage Alliance last year—a nonprofit that aims to “re-potty train the world” by breaking taboos in talking about the poop and pee problem, as well as uniting experts from various key sectors to work together to end sewage pollution in coastal areas.
To end this pollution, we have to change the ways we handle our sewage. Even more exciting is that by solving the sewage problem we can create all sorts of economic benefits. In 2015, human poop was valued at $9.5 billion a year globally, which today would be $11.5 billion per year.
What would one do with that sh$t?
We could convert it into high-value goods. Sewage can be used to generate electricity, fertilizer, and drinking water. The technologies not only exist but are getting better and more efficient all the time. Some exciting examples include biodigesters and urine diversion (or peecycling) systems that can produce fertilizer and biogas, essentially natural gas. The United Nations estimates that the biogas produced from poop could provide electricity for 138 million homes. And the recovered and cleaned water can be used for irrigation, laundry and flushing toilets. It can even be refined to the point that it is safe for drinking water – just ask the folks in Orange County, CA who have been doing so for the last few decades.
How do we deal with all the human-made pollutants in our sewage? There is technology for that too. Called pyrolysis, it heats up sludge to high temperatures in the absence of oxygen, which causes most of the substances to degrade and fall apart.
There are solutions to the problems—as long as we acknowledge that the problems exist. The fact that you are reading this means that you are part of the solution already. The next time you flush your toilet, think about where this output may flow. Does your septic system work properly? Does your local treatment plant discharge raw sewage on rainy days? Can that plant implement newer technologies that can upcycle waste? These questions are part of re-potty training the world, one household at a time. And together, these households are the force that can turn back the toxic sewage tide. And keep our oceans blue.