How Will the New Strains of COVID-19 Affect Our Vaccination Plans?

The mutated strains that first arose in the U.K. and South Africa and have now spread to many countries are prompting urgent studies on the effectiveness of current vaccines to neutralize the new strains.
When the world's first Covid-19 vaccine received regulatory approval in November, it appeared that the end of the pandemic might be near. As one by one, the Pfizer/BioNTech, Moderna, AstraZeneca, and Sputnik V vaccines reported successful Phase III results, the prospect of life without lockdowns and restrictions seemed a tantalizing possibility.
But for scientists with many years' worth of experience in studying how viruses adapt over time, it remained clear that the fight against the SARS-CoV-2 virus was far from over. "The more virus circulates, the more it is likely that mutations occur," said Professor Beate Kampmann, director of the Vaccine Centre at the London School of Hygiene & Tropical Medicine. "It is inevitable that new variants will emerge."
Since the start of the pandemic, dozens of new variants of SARS-CoV-2 – containing different mutations in the viral genome sequence - have appeared as it copies itself while spreading through the human population. The majority of these mutations are inconsequential, but in recent months, some mutations have emerged in the receptor binding domain of the virus's spike protein, increasing how tightly it binds to human cells. These mutations appear to make some new strains up to 70 percent more transmissible, though estimates vary and more lab experiments are needed. Such new strains include the B.1.1.7 variant - currently the dominant strain in the UK – and the 501Y.V2 variant, which was first found in South Africa.
"I'm quite optimistic that even with these mutations, immunity is not going to suddenly fail on us."
Because so many more people are becoming infected with the SARS-CoV-2 virus as a result, vaccinologists point out that these new strains will prolong the pandemic.
"It may take longer to reach vaccine-induced herd immunity," says Deborah Fuller, professor of microbiology at the University of Washington School of Medicine. "With a more transmissible variant taking over, an even larger percentage of the population will need to get vaccinated before we can shut this pandemic down."
That is, of course, as long as the vaccinations are still highly protective. The South African variant, in particular, contains a mutation called E484K that is raising alarms among scientists. Emerging evidence indicates that this mutation allows the virus to escape from some people's immune responses, and thus could potentially weaken the effectiveness of current vaccines.
What We Know So Far
Over the past few weeks, manufacturers of the approved Covid-19 vaccines have been racing to conduct experiments, assessing whether their jabs still work well against the new variants. This process involves taking blood samples from people who have already been vaccinated and assessing whether the antibodies generated by those people can neutralize the new strains in a test tube.
Pfizer has just released results from the first of these studies, declaring that their vaccine was found to still be effective at neutralizing strains of the virus containing the N501Y mutation of the spike protein, one of the mutations present within both the UK and South African variants.
However, the study did not look at the full set of mutations contained within either of these variants. Earlier this week, academics at the Fred Hutchinson Cancer Research Center in Seattle suggested that the E484K spike protein mutation could be most problematic, publishing a study which showed that the efficacy of neutralizing antibodies against this region dropped by more than ten-fold because of the mutation.
Thankfully, this development is not expected to make vaccines useless. One of the Fred Hutch researchers, Jesse Bloom, told STAT News that he did not expect this mutation to seriously reduce vaccine efficacy, and that more harmful mutations would need to accrue over time to pose a very significant threat to vaccinations.
"I'm quite optimistic that even with these mutations, immunity is not going to suddenly fail on us," Bloom told STAT. "It might be gradually eroded, but it's not going to fail on us, at least in the short term."
While further vaccine efficacy data will emerge in the coming weeks, other vaccinologists are keen to stress this same point: At most, there will be a marginal drop in efficacy against the new variants.
"Each vaccine induces what we call polyclonal antibodies targeting multiple parts of the spike protein," said Fuller. "So if one antibody target mutates, there are other antibody targets on the spike protein that could still neutralize the virus. The vaccine platforms also induce T-cell responses that could provide a second line of defense. If some virus gets past antibodies, T-cell responses can find and eliminate infected cells before the virus does too much damage."
She estimates that if vaccine efficacy decreases, for example from 95% to 85%, against one of the new variants, the main implications will be that some individuals who might otherwise have become severely ill, may still experience mild or moderate symptoms from an infection -- but crucially, they will not end up in intensive care.
"Plug and Play" Vaccine Platforms
One of the advantages of the technologies which have been pioneered to create the Covid-19 vaccines is that they are relatively straightforward to update with a new viral sequence. The mRNA technology used in the Pfizer/BioNTech and Moderna vaccines, and the adenovirus vectors used in the Astra Zeneca and Sputnik V vaccines, are known as 'plug and play' platforms, meaning that a new form of the vaccine can be rapidly generated against any emerging variant.
"With a rapid pipeline for manufacture established, these new vaccine technologies could enable production and distribution within 1-3 months of a new variant emerging."
While the technology for the seasonal influenza vaccines is relatively inefficient, requiring scientists to grow and cultivate the new strain in the lab before vaccines can be produced - a process that takes nine months - mRNA and adenovirus-based vaccines can be updated within a matter of weeks. According to BioNTech CEO Uğur Şahin, a new version of their vaccine could be produced in six weeks.
"With a rapid pipeline for manufacture established, these new vaccine technologies could enable production and distribution within 1-3 months of a new variant emerging," says Fuller.
Fuller predicts that more new variants of the virus are almost certain to emerge within the coming months and years, potentially requiring the public to receive booster shots. This means there is one key advantage the mRNA-based vaccines have over the adenovirus technologies. mRNA vaccines only express the spike protein, while the AstraZeneca and Sputnik V vaccines use adenoviruses - common viruses most of us are exposed to - as a delivery mechanism for genes from the SARS-CoV-2 virus.
"For the adenovirus vaccines, our bodies make immune responses against both SARS-CoV-2 and the adenovirus backbone of the vaccine," says Fuller. "That means if you update the adenovirus-based vaccine with the new variant and then try to boost people, they may respond less well to the new vaccine, because they already have antibodies against the adenovirus that could block the vaccine from working. This makes mRNA vaccines more amenable to repeated use."
Regulatory Unknowns
One of the key questions remains whether regulators would require new versions of the vaccine to go through clinical trials, a hurdle which would slow down the response to emerging strains, or whether the seasonal influenza paradigm will be followed, whereby a new form of the vaccine can be released without further clinical testing.
Regulators are currently remaining tight-lipped on which process they will choose to follow, until there is more information on how vaccines respond against the new variants. "Only when such information becomes available can we start the scientific evaluation of what data would be needed to support such a change and assess what regulatory procedure would be required for that," said Rebecca Harding, communications officer for the European Medicines Agency.
The Food and Drug Administration (FDA) did not respond to requests for comment before press time.
While vaccinologists feel it is unlikely that a new complete Phase III trial would be required, some believe that because these are new technologies, regulators may well demand further safety data before approving an updated version of the vaccine.
"I would hope if we ever have to update the current vaccines, regulatory authorities will treat it like influenza," said Drew Weissman, professor of medicine at the University of Pennsylvania, who was involved in developing the mRNA technology behind the Pfizer/BioNTech and Moderna vaccines. "I would guess, at worst, they may want a new Phase 1 or 1 and 2 clinical trials."
Others suggest that rather than new trials, some bridging experiments may suffice to demonstrate that the levels of neutralizing antibodies induced by the new form of the vaccine are comparable to the previous one. "Vaccines have previously been licensed by this kind of immunogenicity data only, for example meningitis vaccines," said Kampmann.
While further mutations and strains of SARS-CoV-2 are inevitable, some scientists are concerned that the vaccine rollout strategy being employed in some countries -- of distributing a first shot to as many people as possible, and potentially delaying second shots as a result -- could encourage more new variants to emerge. Just today, the Biden administration announced its intention to release nearly all vaccine doses on hand right away, without keeping a reserve for second shots. This plan risks relying on vaccine manufacturing to ramp up quickly to keep pace if people are to receive their second shots at the right intervals.
"I am not very happy about this change as it could lead to a large number of people out there with partial immunity and this could select new mutations, and escalate the potential problem of vaccine escape."
The Biden administration's shift appears to conflict with the FDA's recent position that second doses should be given on a strict schedule, without any departure from the three- and four-week intervals established in clinical trials. Two top FDA officials said in a statement that changing the dosing schedule "is premature and not rooted solidly in the available evidence. Without appropriate data supporting such changes in vaccine administration, we run a significant risk of placing public health at risk, undermining the historic vaccination efforts to protect the population from COVID-19."
"I understand the argument of trying to get at least partial protection to as many people as possible, but I am concerned about the increased interval between the doses that is now being proposed," said Kampmann. "I am not very happy about this change as it could lead to a large number of people out there with partial immunity and this could select new mutations, and escalate the potential problem of vaccine escape."
But it's worth emphasizing that the virus is unlikely for now to accumulate enough harmful mutations to render the current vaccines completely ineffective.
"It will be very hard for the virus to evolve to completely evade the antibody responses the vaccines induce," said Fuller. "The parts of the virus that are targeted by vaccine-induced antibodies are essential for the virus to infect our cells. If the virus tries to mutate these parts to evade antibodies, then it could compromise its own fitness or even abort its ability to infect. To be sure, the virus is developing these mutations, but we just don't see these variants emerge because they die out."
Is a Successful HIV Vaccine Finally on the Horizon?
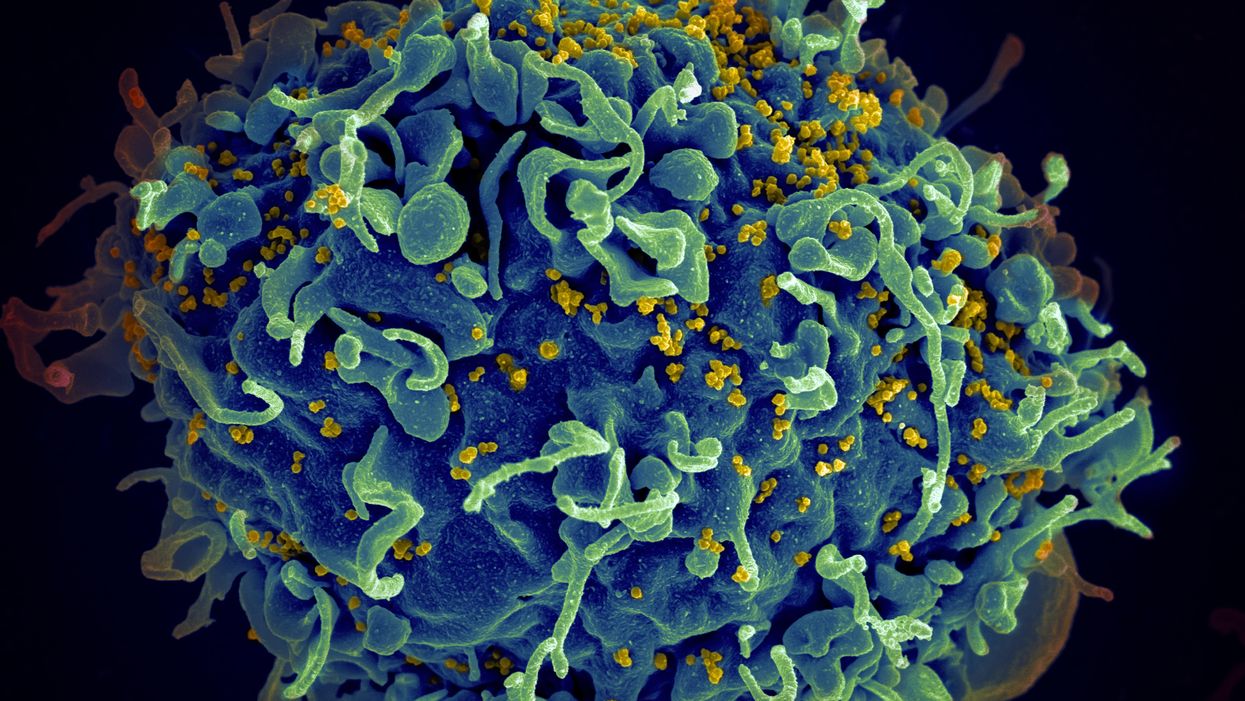
The HIV virus (yellow) infecting a human cell.
Few vaccines have been as complicated—and filled with false starts and crushed hopes—as the development of an HIV vaccine.
While antivirals help HIV-positive patients live longer and reduce viral transmission to virtually nil, these medications must be taken for life, and preventative medications like pre-exposure prophylaxis, known as PrEP, need to be taken every day to be effective. Vaccines, even if they need boosters, would make prevention much easier.
In August, Moderna began human trials for two HIV vaccine candidates based on messenger RNA.
As they have with the Covid-19 pandemic, mRNA vaccines could change the game. The technology could be applied for gene editing therapy, cancer, other infectious diseases—even a universal influenza vaccine.
In the past, three other mRNA vaccines completed phase-2 trials without success. But the easily customizable platforms mean the vaccines can be tweaked better to target HIV as researchers learn more.
Ever since HIV was discovered as the virus causing AIDS, researchers have been searching for a vaccine. But the decades-long journey has so far been fruitless; while some vaccine candidates showed promise in early trials, none of them have worked well among later-stage clinical trials.
There are two main reasons for this: HIV evolves incredibly quickly, and the structure of the virus makes it very difficult to neutralize with antibodies.
"We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
"You know the panic that goes on when a new coronavirus variant surfaces?" asked John Moore, professor of microbiology and immunology at Weill Cornell Medicine who has researched HIV vaccines for 25 years. "With HIV, that kind of variation [happens] pretty much every day in everybody who's infected. It's just orders of magnitude more variable a virus."
Vaccines like these usually work by imitating the outer layer of a virus to teach cells how to recognize and fight off the real thing off before it enters the cell. "If you can prevent landing, you can essentially keep the virus out of the cell," said Larry Corey, the former president and director of the Fred Hutchinson Cancer Research Center who helped run a recent trial of a Johnson & Johnson HIV vaccine candidate, which failed its first efficacy trial.
Like the coronavirus, HIV also has a spike protein with a receptor-binding domain—what Moore calls "the notorious RBD"—that could be neutralized with antibodies. But while that target sticks out like a sore thumb in a virus like SARS-CoV-2, in HIV it's buried under a dense shield. That's not the only target for neutralizing the virus, but all of the targets evolve rapidly and are difficult to reach.
"We understand these targets. We know where they are. But it's still proving incredibly difficult to raise antibodies against them by vaccination," Moore said.
In fact, mRNA vaccines for HIV have been under development for years. The Covid vaccines were built on decades of that research. But it's not as simple as building on this momentum, because of how much more complicated HIV is than SARS-CoV-2, researchers said.
"They haven't succeeded because they were not designed appropriately and haven't been able to induce what is necessary for them to induce," Moore said. "The mRNA technology will enable you to produce a lot of antibodies to the HIV envelope, but if they're the wrong antibodies that doesn't solve the problem."
Part of the problem is that the HIV vaccines have to perform better than our own immune systems. Many vaccines are created by imitating how our bodies overcome an infection, but that doesn't happen with HIV. Once you have the virus, you can't fight it off on your own.
"The human immune system actually does not know how to innately cure HIV," Corey said. "We needed to improve upon the human immune system to make it quicker… with Covid. But we have to actually be better than the human immune system" with HIV.
But in the past few years, there have been impressive leaps in understanding how an HIV vaccine might work. Scientists have known for decades that neutralizing antibodies are key for a vaccine. But in 2010 or so, they were able to mimic the HIV spike and understand how antibodies need to disable the virus. "It helps us understand the nature of the problem, but doesn't instantly solve the problem," Moore said. "Without neutralizing antibodies, you don't have a chance."
Because the vaccines need to induce broadly neutralizing antibodies, and because it's very difficult to neutralize the highly variable HIV, any vaccine will likely be a series of shots that teach the immune system to be on the lookout for a variety of potential attacks.
"Each dose is going to have to have a different purpose," Corey said. "And we hope by the end of the third or fourth dose, we will achieve the level of neutralization that we want."
That's not ideal, because each individual component has to be made and tested—and four shots make the vaccine harder to administer.
"You wouldn't even be going down that route, if there was a better alternative," Moore said. "But there isn't a better alternative."
The mRNA platform is exciting because it is easily customizable, which is especially important in fighting against a shapeshifting, complicated virus. And the mRNA platform has shown itself, in the Covid pandemic, to be safe and quick to make. Effective Covid vaccines were comparatively easy to develop, since the coronavirus is easier to battle than HIV. But companies like Moderna are capitalizing on their success to launch other mRNA therapeutics and vaccines, including the HIV trial.
"You can make the vaccine in two months, three months, in a research lab, and not a year—and the cost of that is really less," Corey said. "It gives us a chance to try many more options, if we've got a good response."
In a trial on macaque monkeys, the Moderna vaccine reduced the chances of infection by 85 percent. "The mRNA platform represents a very promising approach for the development of an HIV vaccine in the future," said Dr. Peng Zhang, who is helping lead the trial at the National Institute of Allergy and Infectious Diseases.
Moderna's trial in humans represents "a very exciting possibility for the prevention of HIV infection," Dr. Monica Gandhi, director of the UCSF-Gladstone Center for AIDS Research, said in an email. "We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
If a successful HIV vaccine is developed, the series of shots could include an mRNA shot that primes the immune system, followed by protein subunits that generate the necessary antibodies, Moore said.
"I think it's the only thing that's worth doing," he said. "Without something complicated like that, you have no chance of inducing broadly neutralizing antibodies."
"I can't guarantee you that's going to work," Moore added. "It may completely fail. But at least it's got some science behind it."
New Podcast: The Lead Scientist for the NASA Mission to Venus
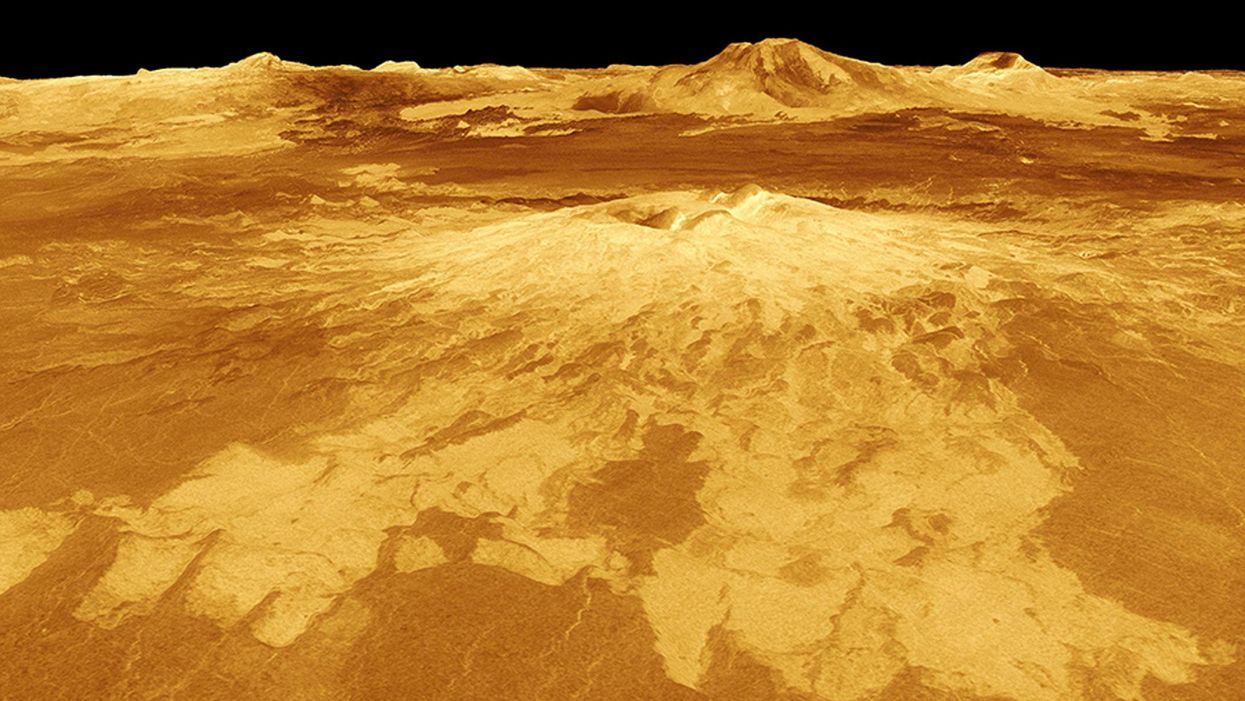
The volcano Sapas Mons is displayed in the center of this computer-generated three-dimensional perspective view of the surface of Venus.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, our guest is JPL's Dr. Suzanne Smrekar, who will be pushing the boundaries of knowledge about the planet Venus during the upcoming VERITAS mission set to launch in 2028. Why did Earth's twin planet develop so differently than our own? Could Venus ever have hosted life? What is the bigger purpose for humanity in studying the solar system -- is it purely scientific, or is it also a matter of art and philosophy? Hear Dr. Smrekar discuss all this and more on the latest episode.
Watch the 30-Second Trailer:
Listen to the Episode:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.