How Will the New Strains of COVID-19 Affect Our Vaccination Plans?

The mutated strains that first arose in the U.K. and South Africa and have now spread to many countries are prompting urgent studies on the effectiveness of current vaccines to neutralize the new strains.
When the world's first Covid-19 vaccine received regulatory approval in November, it appeared that the end of the pandemic might be near. As one by one, the Pfizer/BioNTech, Moderna, AstraZeneca, and Sputnik V vaccines reported successful Phase III results, the prospect of life without lockdowns and restrictions seemed a tantalizing possibility.
But for scientists with many years' worth of experience in studying how viruses adapt over time, it remained clear that the fight against the SARS-CoV-2 virus was far from over. "The more virus circulates, the more it is likely that mutations occur," said Professor Beate Kampmann, director of the Vaccine Centre at the London School of Hygiene & Tropical Medicine. "It is inevitable that new variants will emerge."
Since the start of the pandemic, dozens of new variants of SARS-CoV-2 – containing different mutations in the viral genome sequence - have appeared as it copies itself while spreading through the human population. The majority of these mutations are inconsequential, but in recent months, some mutations have emerged in the receptor binding domain of the virus's spike protein, increasing how tightly it binds to human cells. These mutations appear to make some new strains up to 70 percent more transmissible, though estimates vary and more lab experiments are needed. Such new strains include the B.1.1.7 variant - currently the dominant strain in the UK – and the 501Y.V2 variant, which was first found in South Africa.
"I'm quite optimistic that even with these mutations, immunity is not going to suddenly fail on us."
Because so many more people are becoming infected with the SARS-CoV-2 virus as a result, vaccinologists point out that these new strains will prolong the pandemic.
"It may take longer to reach vaccine-induced herd immunity," says Deborah Fuller, professor of microbiology at the University of Washington School of Medicine. "With a more transmissible variant taking over, an even larger percentage of the population will need to get vaccinated before we can shut this pandemic down."
That is, of course, as long as the vaccinations are still highly protective. The South African variant, in particular, contains a mutation called E484K that is raising alarms among scientists. Emerging evidence indicates that this mutation allows the virus to escape from some people's immune responses, and thus could potentially weaken the effectiveness of current vaccines.
What We Know So Far
Over the past few weeks, manufacturers of the approved Covid-19 vaccines have been racing to conduct experiments, assessing whether their jabs still work well against the new variants. This process involves taking blood samples from people who have already been vaccinated and assessing whether the antibodies generated by those people can neutralize the new strains in a test tube.
Pfizer has just released results from the first of these studies, declaring that their vaccine was found to still be effective at neutralizing strains of the virus containing the N501Y mutation of the spike protein, one of the mutations present within both the UK and South African variants.
However, the study did not look at the full set of mutations contained within either of these variants. Earlier this week, academics at the Fred Hutchinson Cancer Research Center in Seattle suggested that the E484K spike protein mutation could be most problematic, publishing a study which showed that the efficacy of neutralizing antibodies against this region dropped by more than ten-fold because of the mutation.
Thankfully, this development is not expected to make vaccines useless. One of the Fred Hutch researchers, Jesse Bloom, told STAT News that he did not expect this mutation to seriously reduce vaccine efficacy, and that more harmful mutations would need to accrue over time to pose a very significant threat to vaccinations.
"I'm quite optimistic that even with these mutations, immunity is not going to suddenly fail on us," Bloom told STAT. "It might be gradually eroded, but it's not going to fail on us, at least in the short term."
While further vaccine efficacy data will emerge in the coming weeks, other vaccinologists are keen to stress this same point: At most, there will be a marginal drop in efficacy against the new variants.
"Each vaccine induces what we call polyclonal antibodies targeting multiple parts of the spike protein," said Fuller. "So if one antibody target mutates, there are other antibody targets on the spike protein that could still neutralize the virus. The vaccine platforms also induce T-cell responses that could provide a second line of defense. If some virus gets past antibodies, T-cell responses can find and eliminate infected cells before the virus does too much damage."
She estimates that if vaccine efficacy decreases, for example from 95% to 85%, against one of the new variants, the main implications will be that some individuals who might otherwise have become severely ill, may still experience mild or moderate symptoms from an infection -- but crucially, they will not end up in intensive care.
"Plug and Play" Vaccine Platforms
One of the advantages of the technologies which have been pioneered to create the Covid-19 vaccines is that they are relatively straightforward to update with a new viral sequence. The mRNA technology used in the Pfizer/BioNTech and Moderna vaccines, and the adenovirus vectors used in the Astra Zeneca and Sputnik V vaccines, are known as 'plug and play' platforms, meaning that a new form of the vaccine can be rapidly generated against any emerging variant.
"With a rapid pipeline for manufacture established, these new vaccine technologies could enable production and distribution within 1-3 months of a new variant emerging."
While the technology for the seasonal influenza vaccines is relatively inefficient, requiring scientists to grow and cultivate the new strain in the lab before vaccines can be produced - a process that takes nine months - mRNA and adenovirus-based vaccines can be updated within a matter of weeks. According to BioNTech CEO Uğur Şahin, a new version of their vaccine could be produced in six weeks.
"With a rapid pipeline for manufacture established, these new vaccine technologies could enable production and distribution within 1-3 months of a new variant emerging," says Fuller.
Fuller predicts that more new variants of the virus are almost certain to emerge within the coming months and years, potentially requiring the public to receive booster shots. This means there is one key advantage the mRNA-based vaccines have over the adenovirus technologies. mRNA vaccines only express the spike protein, while the AstraZeneca and Sputnik V vaccines use adenoviruses - common viruses most of us are exposed to - as a delivery mechanism for genes from the SARS-CoV-2 virus.
"For the adenovirus vaccines, our bodies make immune responses against both SARS-CoV-2 and the adenovirus backbone of the vaccine," says Fuller. "That means if you update the adenovirus-based vaccine with the new variant and then try to boost people, they may respond less well to the new vaccine, because they already have antibodies against the adenovirus that could block the vaccine from working. This makes mRNA vaccines more amenable to repeated use."
Regulatory Unknowns
One of the key questions remains whether regulators would require new versions of the vaccine to go through clinical trials, a hurdle which would slow down the response to emerging strains, or whether the seasonal influenza paradigm will be followed, whereby a new form of the vaccine can be released without further clinical testing.
Regulators are currently remaining tight-lipped on which process they will choose to follow, until there is more information on how vaccines respond against the new variants. "Only when such information becomes available can we start the scientific evaluation of what data would be needed to support such a change and assess what regulatory procedure would be required for that," said Rebecca Harding, communications officer for the European Medicines Agency.
The Food and Drug Administration (FDA) did not respond to requests for comment before press time.
While vaccinologists feel it is unlikely that a new complete Phase III trial would be required, some believe that because these are new technologies, regulators may well demand further safety data before approving an updated version of the vaccine.
"I would hope if we ever have to update the current vaccines, regulatory authorities will treat it like influenza," said Drew Weissman, professor of medicine at the University of Pennsylvania, who was involved in developing the mRNA technology behind the Pfizer/BioNTech and Moderna vaccines. "I would guess, at worst, they may want a new Phase 1 or 1 and 2 clinical trials."
Others suggest that rather than new trials, some bridging experiments may suffice to demonstrate that the levels of neutralizing antibodies induced by the new form of the vaccine are comparable to the previous one. "Vaccines have previously been licensed by this kind of immunogenicity data only, for example meningitis vaccines," said Kampmann.
While further mutations and strains of SARS-CoV-2 are inevitable, some scientists are concerned that the vaccine rollout strategy being employed in some countries -- of distributing a first shot to as many people as possible, and potentially delaying second shots as a result -- could encourage more new variants to emerge. Just today, the Biden administration announced its intention to release nearly all vaccine doses on hand right away, without keeping a reserve for second shots. This plan risks relying on vaccine manufacturing to ramp up quickly to keep pace if people are to receive their second shots at the right intervals.
"I am not very happy about this change as it could lead to a large number of people out there with partial immunity and this could select new mutations, and escalate the potential problem of vaccine escape."
The Biden administration's shift appears to conflict with the FDA's recent position that second doses should be given on a strict schedule, without any departure from the three- and four-week intervals established in clinical trials. Two top FDA officials said in a statement that changing the dosing schedule "is premature and not rooted solidly in the available evidence. Without appropriate data supporting such changes in vaccine administration, we run a significant risk of placing public health at risk, undermining the historic vaccination efforts to protect the population from COVID-19."
"I understand the argument of trying to get at least partial protection to as many people as possible, but I am concerned about the increased interval between the doses that is now being proposed," said Kampmann. "I am not very happy about this change as it could lead to a large number of people out there with partial immunity and this could select new mutations, and escalate the potential problem of vaccine escape."
But it's worth emphasizing that the virus is unlikely for now to accumulate enough harmful mutations to render the current vaccines completely ineffective.
"It will be very hard for the virus to evolve to completely evade the antibody responses the vaccines induce," said Fuller. "The parts of the virus that are targeted by vaccine-induced antibodies are essential for the virus to infect our cells. If the virus tries to mutate these parts to evade antibodies, then it could compromise its own fitness or even abort its ability to infect. To be sure, the virus is developing these mutations, but we just don't see these variants emerge because they die out."
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
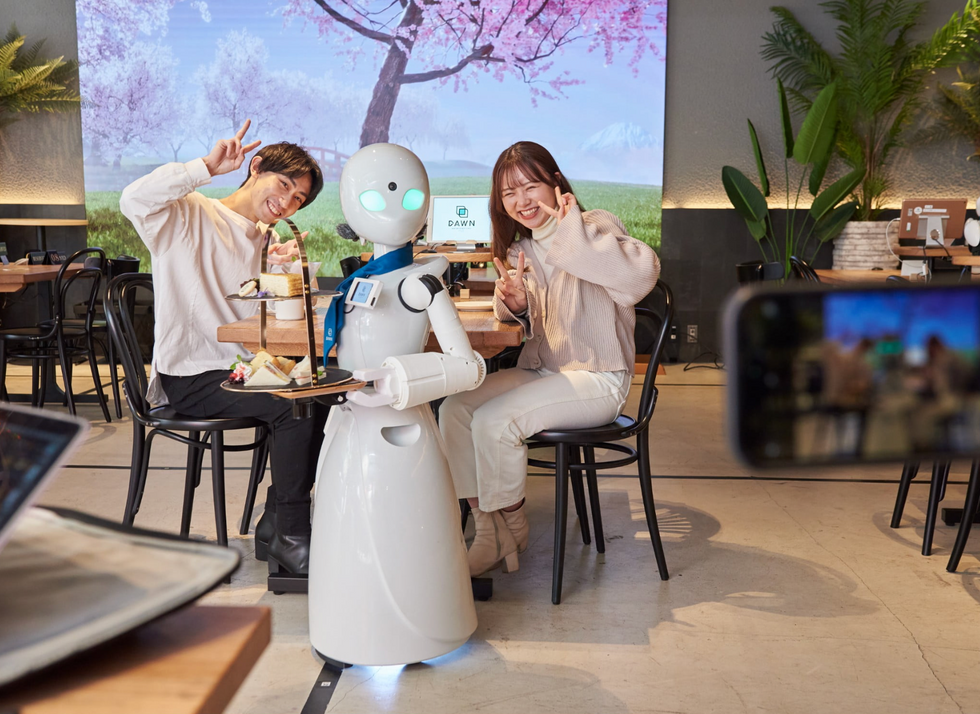
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
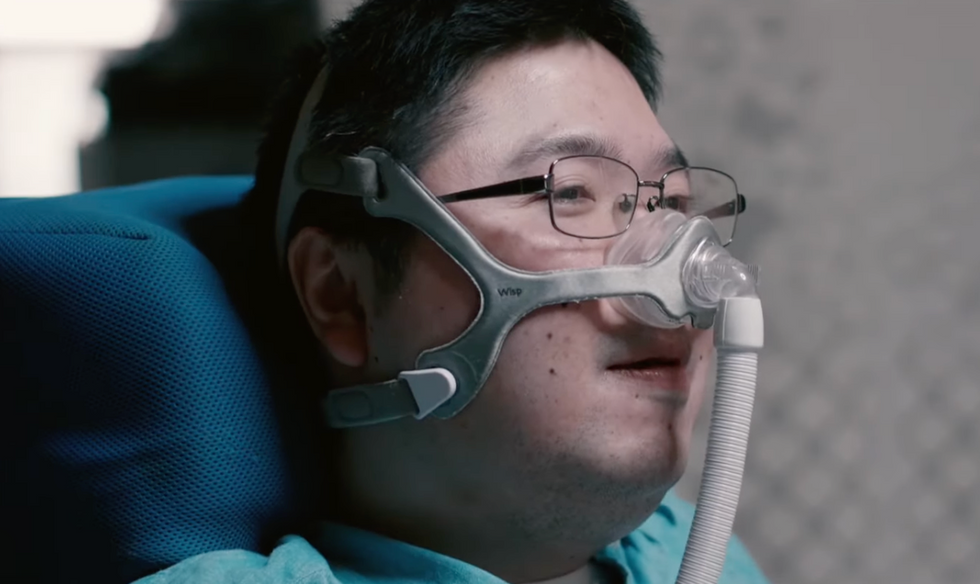
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

