Scientists Are Growing an Edible Cholera Vaccine in Rice

The world's attention has been focused on the coronavirus crisis but Yemen, Bangladesh and many others countries in Asia and Africa are also in the grips of another pandemic: cholera. The current cholera pandemic first emerged in the 1970s and has devastated many communities in low-income countries. Each year, cholera is responsible for an estimated 1.3 million to 4 million cases and 21,000 to 143,000 deaths worldwide.
Immunologist Hiroshi Kiyono and his team at the University of Tokyo hope they can be part of the solution: They're making a cholera vaccine out of rice.
"It is much less expensive than a traditional vaccine, by a long shot."
Cholera is caused by eating food or drinking water that's contaminated by the feces of a person infected with the cholera bacteria, Vibrio cholerae. The bacteria produces the cholera toxin in the intestines, leading to vomiting, diarrhea and severe dehydration. Cholera can kill within hours of infection if it if's not treated quickly.
Current cholera vaccines are mainly oral. The most common oral are given in two doses and are made out of animal or insect cells that are infected with killed or weakened cholera bacteria. Dukoral also includes cells infected with CTB, a non-harmful part of the cholera toxin. Scientists grow cells containing the cholera bacteria and the CTB in bioreactors, large tanks in which conditions can be carefully controlled.
These cholera vaccines offer moderate protection but it wears off relatively quickly. Cold storage can also be an issue. The most common oral vaccines can be stored at room temperature but only for 14 days.
"Current vaccines confer around 60% efficacy over five years post-vaccination," says Lucy Breakwell, who leads the U.S. Centers for Disease Control and Prevention's cholera work within Global Immunization Division. Given the limited protection, refrigeration issue, and the fact that current oral vaccines require two disease, delivery of cholera vaccines in a campaign or emergency setting can be challenging. "There is a need to develop and test new vaccines to improve public health response to cholera outbreaks."
A New Kind of Vaccine
Kiyono and scientists at Tokyo University are creating a new, plant-based cholera vaccine dubbed MucoRice-CTB. The researchers genetically modify rice so that it contains CTB, a non-harmful part of the cholera toxin. The rice is crushed into a powder, mixed with saline solution and then drunk. The digestive tract is lined with mucosal membranes which contain the mucosal immune system. The mucosal immune system gets trained to recognize the cholera toxin as the rice passes through the intestines.
The cholera toxin has two main parts: the A subunit, which is harmful, and the B subunit, also known as CTB, which is nontoxic but allows the cholera bacteria to attach to gut cells. By inducing CTB-specific antibodies, "we might be able to block the binding of the vaccine toxin to gut cells, leading to the prevention of the toxin causing diarrhea," Kiyono says.
Kiyono studies the immune responses that occur at mucosal membranes across the body. He chose to focus on cholera because he wanted to replicate the way traditional vaccines work to get mucosal membranes in the digestive tract to produce an immune response. The difference is that his team is creating a food-based vaccine to induce this immune response. They are also solely focusing on getting the vaccine to induce antibodies for the cholera toxin. Since the cholera toxin is responsible for bacteria sticking to gut cells, the hope is that they can stop this process by producing antibodies for the cholera toxin. Current cholera vaccines target the cholera bacteria or both the bacteria and the toxin.
David Pascual, an expert in infectious diseases and immunology at the University of Florida, thinks that the MucoRice vaccine has huge promise. "I truly believe that the development of a food-based vaccine can be effective. CTB has a natural affinity for sampling cells in the gut to adhere, be processed, and then stimulate our immune system, he says. "In addition to vaccinating the gut, MucoRice has the potential to touch other mucosal surfaces in the mouth, which can help generate an immune response locally in the mouth and distally in the gut."
Cost Effectiveness
Kiyono says the MucoRice vaccine is much cheaper to produce than a traditional vaccine. Current vaccines need expensive bioreactors to grow cell cultures under very controlled, sterile conditions. This makes them expensive to manufacture, as different types of cell cultures need to be grown in separate buildings to avoid any chance of contamination. MucoRice doesn't require such an expensive manufacturing process because the rice plants themselves act as bioreactors.
The MucoRice vaccine also doesn't require the high cost of cold storage. It can be stored at room temperature for up to three years unlike traditional vaccines. "Plant-based vaccine development platforms present an exciting tool to reduce vaccine manufacturing costs, expand vaccine shelf life, and remove refrigeration requirements, all of which are factors that can limit vaccine supply and accessibility," Breakwell says.
Kathleen Hefferon, a microbiologist at Cornell University agrees. "It is much less expensive than a traditional vaccine, by a long shot," she says. "The fact that it is made in rice means the vaccine can be stored for long periods on the shelf, without losing its activity."
A plant-based vaccine may even be able to address vaccine hesitancy, which has become a growing problem in recent years. Hefferon suggests that "using well-known food plants may serve to reduce the anxiety of some vaccine hesitant people."
Challenges of Plant Vaccines
Despite their advantages, no plant-based vaccines have been commercialized for human use. There are a number of reasons for this, ranging from the potential for too much variation in plants to the lack of facilities large enough to grow crops that comply with good manufacturing practices. Several plant vaccines for diseases like HIV and COVID-19 are in development, but they're still in early stages.
In developing the MucoRice vaccine, scientists at the University of Tokyo have tried to overcome some of the problems with plant vaccines. They've created a closed facility where they can grow rice plants directly in nutrient-rich water rather than soil. This ensures they can grow crops all year round in a space that satisfies regulations. There's also less chance for variation since the environment is tightly controlled.
Clinical Trials and Beyond
After successfully growing rice plants containing the vaccine, the team carried out their first clinical trial. It was completed early this year. Thirty participants received a placebo and 30 received the vaccine. They were all Japanese men between the ages of 20 and 40 years old. 60 percent produced antibodies against the cholera toxin with no side effects. It was a promising result. However, there are still some issues Kiyono's team need to address.
The vaccine may not provide enough protection on its own. The antigen in any vaccine is the substance it contains to induce an immune response. For the MucoRice vaccine, the antigen is not the cholera bacteria itself but the cholera toxin the bacteria produces.
"The development of the antigen in rice is innovative," says David Sack, a professor at John Hopkins University and expert in cholera vaccine development. "But antibodies against only the toxin have not been very protective. The major protective antigen is thought to be the LPS." LPS, or lipopolysaccharide, is a component of the outer wall of the cholera bacteria that plays an important role in eliciting an immune response.
The Japanese team is considering getting the rice to also express the O antigen, a core part of the LPS. Further investigation and clinical trials will look into improving the vaccine's efficacy.
Beyond cholera, Kiyono hopes that the vaccine platform could one day be used to make cost-effective vaccines for other pathogens, such as norovirus or coronavirus.
"We believe the MucoRice system may become a new generation of vaccine production, storage, and delivery system."
A New Test Aims to Objectively Measure Pain. It Could Help Legitimate Sufferers Access the Meds They Need.
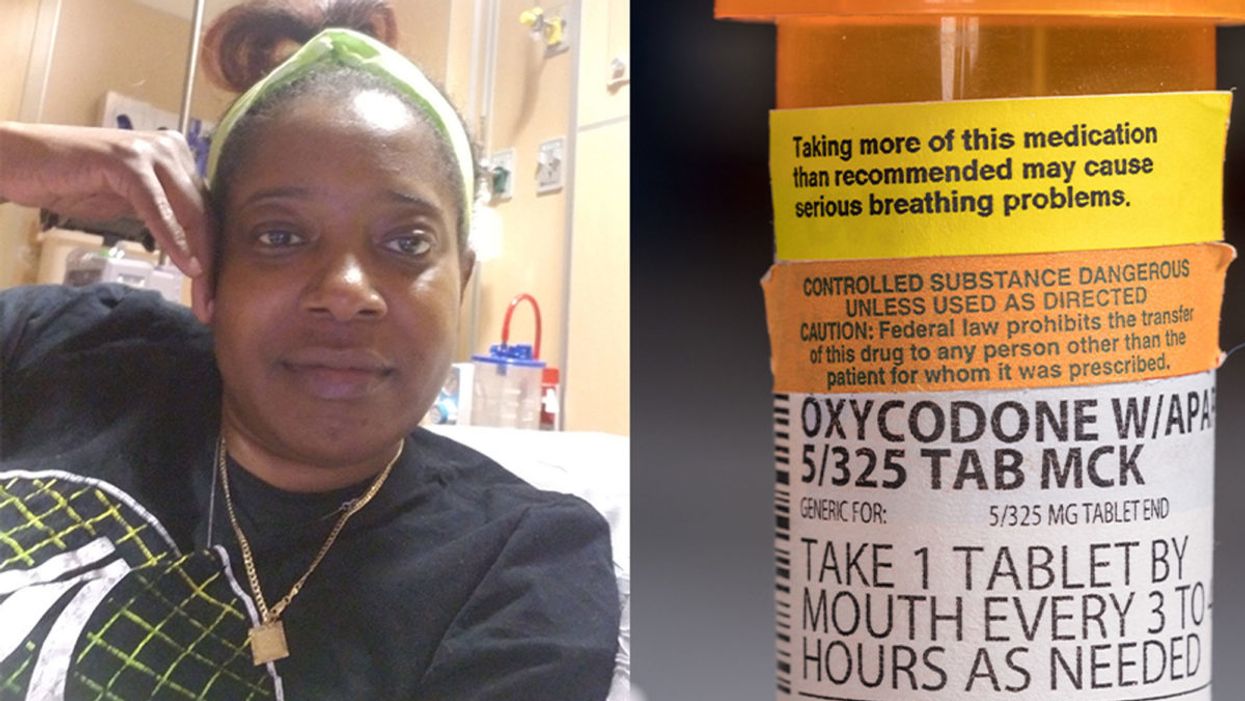
Sickle cell patient Bridgett Willkie found herself being labeled an addict when she sought an opioid prescription to control her pain.
"That throbbing you feel for the first minute after a door slams on your finger."
This is how Central Florida resident Bridgett Willkie describes the attacks of pain caused by her sickle cell anemia – a genetic blood disorder in which a patient's red blood cells become shaped like sickles and get stuck in blood vessels, thereby obstructing the flow of blood and oxygen.
"I found myself being labeled as an addict and I never was."
Willkie's lifelong battle with the condition has led to avascular necrosis in both of her shoulders, hips, knees and ankles. This means that her bone tissue is dying due to insufficient blood supply (sickle cell anemia is among the medical conditions that can decrease blood flow to one's bones).
"That adds to the pain significantly," she says. "Every time my heart beats, it hurts. And the pain moves. It follows the path of circulation. I liken it to a traffic jam in my veins."
For more than a decade, she received prescriptions for Oxycontin. Then, four years ago, her hematologist – who had been her doctor for 18 years – suffered a fatal heart attack. She says her longtime doctor's replacement lacked experience treating sickle cell patients and was uncomfortable writing her a prescription for opioids. What's more, this new doctor wanted to place her in a drug rehab facility.
"Because I refused to go, he stopped writing my scripts," she says. The ensuing three months were spent at home, detoxing. She describes the pain as unbearable. "Sometimes I just wanted to die."
One of the effects of the opioid epidemic is that many legitimate pain patients have seen their opioids significantly reduced or downright discontinued because of their doctors' fears of over-prescribing addictive medications.
"I found myself being labeled as an addict and I never was...Being treated like a drug-seeking patient is degrading and humiliating," says Willkie, who adds that when she is at the hospital, "it's exhausting arguing with the doctors...You dread them making their rounds because every day they come in talking about weaning you off your meds."
Situations such as these are fraught with tension between patients and doctors, who must remain wary about the risk of over-prescribing powerful and addictive medications. Adding to the complexity is that it can be very difficult to reliably assess a patient's level of physical pain.
However, this difficulty may soon decline, as Indiana University School of Medicine researchers, led by Dr. Alexander B. Niculescu, have reportedly devised a way to objectively assess physical pain by analyzing biomarkers in a patient's blood sample. The results of a study involving more than 300 participants were published earlier this year in the journal Molecular Psychiatry.
Niculescu – who is both a professor of psychiatry and medical neuroscience at the IU School of Medicine – explains that, when someone is in severe physical pain, a blood sample will show biomarkers related to intracellular adhesion and cell-signaling mechanisms. He adds that some of these biomarkers "have prior convergent evidence from animal or human studies for involvement in pain."
Aside from reliably measuring pain severity, Niculescu says blood biomarkers can measure the degree of one's response to treatment and also assess the risk of future recurrences of pain. He believes this new method's greatest benefit, however, might be the ability to identify a number of non-opioid medications that a particular patient is likely to respond to, based on his or her biomarker profile.
Clearly, such a method could be a gamechanger for pain patients and the professionals who treat them. As of yet, health workers have been forced to make crucial decisions based on their clinical impressions of patients; such impressions are invariably subjective. A method that enables people to prove the extent of their pain could remove the stigma that many legitimate pain patients face when seeking to obtain their needed medicine. It would also improve their chances of receiving sufficient treatment.
Niculescu says it's "theoretically possible" that there are some conditions which, despite being severe, might not reveal themselves through his testing method. But he also says that, "even if the same molecular markers that are involved in the pain process are not reflected in the blood, there are other indirect markers that should reflect the distress."
Niculescu expects his testing method will be available to the medical community at large within one to three years.
Willkie says she would welcome a reliable pain assessment method. Well-aware that she is not alone in her plight, she has more than 500 Facebook friends with sickle cell disease, and she says that "all of their opioid meds have been restricted or cut" as a result of the opioid crisis. Some now feel compelled to find their opioids "on the streets." She says she personally has never obtained opioids this way. Instead, she relies on marijuana to mitigate her pain.
Niculescu expects his testing method will be available to the medical community at large within one to three years: "It takes a while for things to translate from a lab setting to a commercial testing arena."
In the meantime, for Willkie and other patients, "we have to convince doctors and nurses that we're in pain."
Some people can eat red meat without negative health consequences, which may be due to variability between people's gut microbes.
In different countries' national dietary guidelines, red meats (beef, pork, and lamb) are often confined to a very small corner. Swedish officials, for example, advise the population to "eat less red and processed meat". Experts in Greece recommend consuming no more than four servings of red meat — not per week, but per month.
"Humans 100% rely on the microbes to digest this food."
Yet somehow, the matter is far from settled. Quibbles over the scientific evidence emerge on a regular basis — as in a recent BMJ article titled, "No need to cut red meat, say new guidelines." News headlines lately have declared that limiting red meat may be "bad advice," while carnivore diet enthusiasts boast about the weight loss and good health they've achieved on an all-meat diet. The wildly successful plant-based burgers? To them, a gimmick. The burger wars are on.
Nutrition science would seem the best place to look for answers on the health effects of specific foods. And on one hand, the science is rather clear: in large populations, people who eat more red meat tend to have more health problems, including cardiovascular disease, colorectal cancer, and other conditions. But this sort of correlational evidence fails to settle the matter once and for all; many who look closely at these studies cite methodological shortcomings and a low certainty of evidence.
Some scientists, meanwhile, are trying to cut through the noise by increasing their focus on the mechanisms: exactly how red meat is digested and the step-by-step of how this affects human health. And curiously, as these lines of evidence emerge, several of them center around gut microbes as active participants in red meat's ultimate effects on human health.
Dr. Stanley Hazen, researcher and medical director of preventive cardiology at Cleveland Clinic, was one of the first to zero in on gut microorganisms as possible contributors to the health effects of red meat. In looking for chemical compounds in the blood that could predict the future development of cardiovascular disease, his lab identified a molecule called trimethylamine-N-oxide (TMAO). Little by little, he and his colleagues began to gather both human and animal evidence that TMAO played a role in causing heart disease.
Naturally, they tried to figure out where the TMAO came from. Hazen says, "We found that animal products, and especially red meat, were a dietary source that, [along with] gut microbes, would generate this product that leads to heart disease development." They observed that the gut microbes were essential for making TMAO out of dietary compounds (like red meat) that contained its precursor, trimethylamine (TMA).
So in linking red meat to cardiovascular disease through TMAO, the surprising conclusion, says Hazen, was that, "Without a doubt, [the microbes] are the most important aspect of the whole pathway."
"I think it's just a matter of time [before] we will have therapeutic interventions that actually target our gut microbes, just like the way we take drugs that lower cholesterol levels."
Other researchers have taken an interest in different red-meat-associated health problems, like colorectal cancer and the inflammation that accompanies it. This was the mechanistic link tackled by the lab of professor Karsten Zengler of the UC San Diego Departments of Pediatrics and Bioengineering—and it also led straight back to the gut microbes.
Zengler and colleagues recently published a paper in Nature Microbiology that focused on the effects of a red meat carbohydrate (or sugar) called Neu5Gc.
He explains, "If you eat animal proteins in your diet… the bound sugars in your diet are cleaved off in your gut and they get recycled. Your own cells will not recognize between the foreign sugars and your own sugars, because they look almost identical." The unsuspecting human cells then take up these foreign sugars — spurring antibody production and creating inflammation.
Zengler showed, however, that gut bacteria use enzymes to cleave off the sugar during digestion, stopping the inflammation and rendering the sugar harmless. "There's no enzyme in the human body that can cleave this [sugar] off. Humans 100% rely on the microbes to digest this food," he says.
Both researchers are quick to caution that the health effects of diet are complex. Other work indicates, for example, that while intake of red meat can affect TMAO levels, so can intake of fish and seafood. But these new lines of evidence could help explain why some people, ironically, seem to be in perfect health despite eating a lot of red meat: their ideal frequency of meat consumption may depend on their existing community of gut microbes.
"It helps explain what accounts for inter-person variability," Hazen says.
These emerging mechanisms reinforce overall why it's prudent to limit red meat, just as the nutritional guidelines advised in the first place. But both Hazen and Zengler predict that interventions to buffer the effects of too many ribeyes may be just around the corner.
Zengler says, "Our idea is that you basically can help your own digestive system detoxify these inflammatory compounds in meat, if you continue eating red meat or you want to eat a high amount of red meat." A possibly strategy, he says, is to use specific pre- or probiotics to cultivate an inflammation-reducing gut microbial community.
Hazen foresees the emergence of drugs that act not on the human, but on the human's gut microorganisms. "I think it's just a matter of time [before] we will have therapeutic interventions that actually target our gut microbes, just like the way we take drugs that lower cholesterol levels."
He adds, "It's a matter of 'stay tuned', I think."