Scientists Are Growing an Edible Cholera Vaccine in Rice

The world's attention has been focused on the coronavirus crisis but Yemen, Bangladesh and many others countries in Asia and Africa are also in the grips of another pandemic: cholera. The current cholera pandemic first emerged in the 1970s and has devastated many communities in low-income countries. Each year, cholera is responsible for an estimated 1.3 million to 4 million cases and 21,000 to 143,000 deaths worldwide.
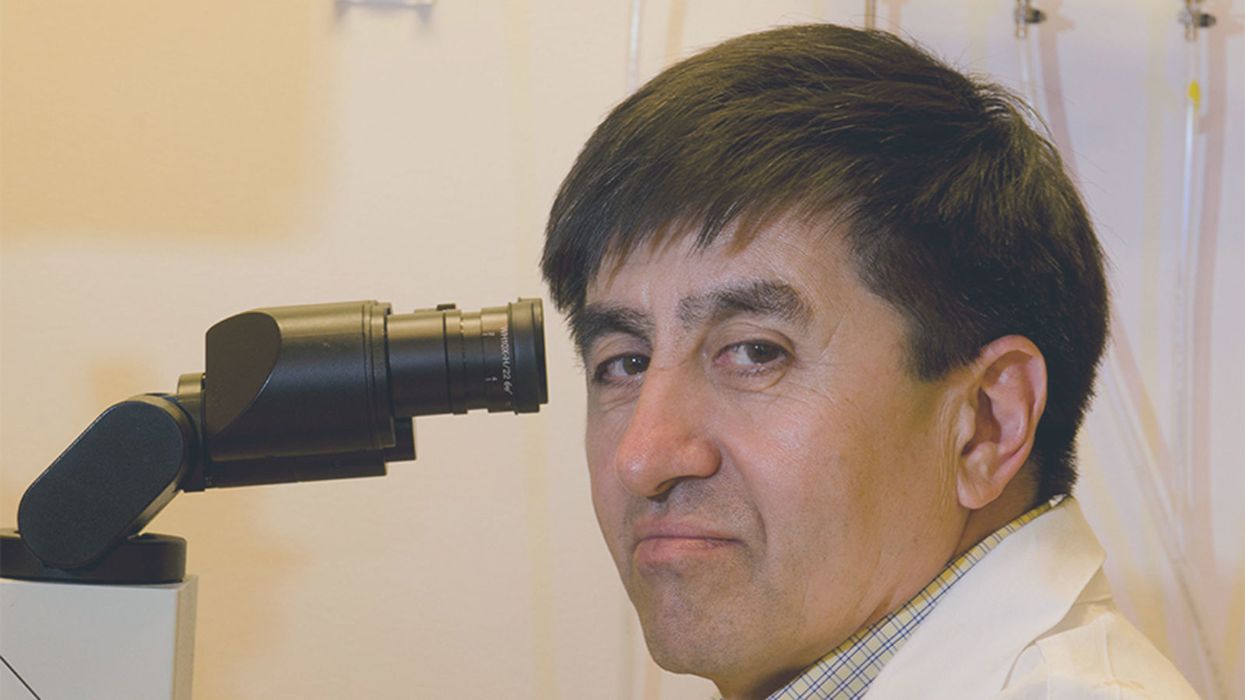
Immunologist Hiroshi Kiyono and his team at the University of Tokyo hope they can be part of the solution: They're making a cholera vaccine out of rice.
"It is much less expensive than a traditional vaccine, by a long shot."
Cholera is caused by eating food or drinking water that's contaminated by the feces of a person infected with the cholera bacteria, Vibrio cholerae. The bacteria produces the cholera toxin in the intestines, leading to vomiting, diarrhea and severe dehydration. Cholera can kill within hours of infection if it if's not treated quickly.
Current cholera vaccines are mainly oral. The most common oral are given in two doses and are made out of animal or insect cells that are infected with killed or weakened cholera bacteria. Dukoral also includes cells infected with CTB, a non-harmful part of the cholera toxin. Scientists grow cells containing the cholera bacteria and the CTB in bioreactors, large tanks in which conditions can be carefully controlled.
These cholera vaccines offer moderate protection but it wears off relatively quickly. Cold storage can also be an issue. The most common oral vaccines can be stored at room temperature but only for 14 days.
"Current vaccines confer around 60% efficacy over five years post-vaccination," says Lucy Breakwell, who leads the U.S. Centers for Disease Control and Prevention's cholera work within Global Immunization Division. Given the limited protection, refrigeration issue, and the fact that current oral vaccines require two disease, delivery of cholera vaccines in a campaign or emergency setting can be challenging. "There is a need to develop and test new vaccines to improve public health response to cholera outbreaks."
A New Kind of Vaccine
Kiyono and scientists at Tokyo University are creating a new, plant-based cholera vaccine dubbed MucoRice-CTB. The researchers genetically modify rice so that it contains CTB, a non-harmful part of the cholera toxin. The rice is crushed into a powder, mixed with saline solution and then drunk. The digestive tract is lined with mucosal membranes which contain the mucosal immune system. The mucosal immune system gets trained to recognize the cholera toxin as the rice passes through the intestines.
The cholera toxin has two main parts: the A subunit, which is harmful, and the B subunit, also known as CTB, which is nontoxic but allows the cholera bacteria to attach to gut cells. By inducing CTB-specific antibodies, "we might be able to block the binding of the vaccine toxin to gut cells, leading to the prevention of the toxin causing diarrhea," Kiyono says.
Kiyono studies the immune responses that occur at mucosal membranes across the body. He chose to focus on cholera because he wanted to replicate the way traditional vaccines work to get mucosal membranes in the digestive tract to produce an immune response. The difference is that his team is creating a food-based vaccine to induce this immune response. They are also solely focusing on getting the vaccine to induce antibodies for the cholera toxin. Since the cholera toxin is responsible for bacteria sticking to gut cells, the hope is that they can stop this process by producing antibodies for the cholera toxin. Current cholera vaccines target the cholera bacteria or both the bacteria and the toxin.
David Pascual, an expert in infectious diseases and immunology at the University of Florida, thinks that the MucoRice vaccine has huge promise. "I truly believe that the development of a food-based vaccine can be effective. CTB has a natural affinity for sampling cells in the gut to adhere, be processed, and then stimulate our immune system, he says. "In addition to vaccinating the gut, MucoRice has the potential to touch other mucosal surfaces in the mouth, which can help generate an immune response locally in the mouth and distally in the gut."
Cost Effectiveness
Kiyono says the MucoRice vaccine is much cheaper to produce than a traditional vaccine. Current vaccines need expensive bioreactors to grow cell cultures under very controlled, sterile conditions. This makes them expensive to manufacture, as different types of cell cultures need to be grown in separate buildings to avoid any chance of contamination. MucoRice doesn't require such an expensive manufacturing process because the rice plants themselves act as bioreactors.
The MucoRice vaccine also doesn't require the high cost of cold storage. It can be stored at room temperature for up to three years unlike traditional vaccines. "Plant-based vaccine development platforms present an exciting tool to reduce vaccine manufacturing costs, expand vaccine shelf life, and remove refrigeration requirements, all of which are factors that can limit vaccine supply and accessibility," Breakwell says.
Kathleen Hefferon, a microbiologist at Cornell University agrees. "It is much less expensive than a traditional vaccine, by a long shot," she says. "The fact that it is made in rice means the vaccine can be stored for long periods on the shelf, without losing its activity."
A plant-based vaccine may even be able to address vaccine hesitancy, which has become a growing problem in recent years. Hefferon suggests that "using well-known food plants may serve to reduce the anxiety of some vaccine hesitant people."
Challenges of Plant Vaccines
Despite their advantages, no plant-based vaccines have been commercialized for human use. There are a number of reasons for this, ranging from the potential for too much variation in plants to the lack of facilities large enough to grow crops that comply with good manufacturing practices. Several plant vaccines for diseases like HIV and COVID-19 are in development, but they're still in early stages.
In developing the MucoRice vaccine, scientists at the University of Tokyo have tried to overcome some of the problems with plant vaccines. They've created a closed facility where they can grow rice plants directly in nutrient-rich water rather than soil. This ensures they can grow crops all year round in a space that satisfies regulations. There's also less chance for variation since the environment is tightly controlled.
Clinical Trials and Beyond
After successfully growing rice plants containing the vaccine, the team carried out their first clinical trial. It was completed early this year. Thirty participants received a placebo and 30 received the vaccine. They were all Japanese men between the ages of 20 and 40 years old. 60 percent produced antibodies against the cholera toxin with no side effects. It was a promising result. However, there are still some issues Kiyono's team need to address.
The vaccine may not provide enough protection on its own. The antigen in any vaccine is the substance it contains to induce an immune response. For the MucoRice vaccine, the antigen is not the cholera bacteria itself but the cholera toxin the bacteria produces.
"The development of the antigen in rice is innovative," says David Sack, a professor at John Hopkins University and expert in cholera vaccine development. "But antibodies against only the toxin have not been very protective. The major protective antigen is thought to be the LPS." LPS, or lipopolysaccharide, is a component of the outer wall of the cholera bacteria that plays an important role in eliciting an immune response.
The Japanese team is considering getting the rice to also express the O antigen, a core part of the LPS. Further investigation and clinical trials will look into improving the vaccine's efficacy.
Beyond cholera, Kiyono hopes that the vaccine platform could one day be used to make cost-effective vaccines for other pathogens, such as norovirus or coronavirus.
"We believe the MucoRice system may become a new generation of vaccine production, storage, and delivery system."
Dr. Shoukhrat Mitalipov, May 13, 2013.
Biologist Shoukhrat Mitalipov is famous—and controversial--in the world of cutting-edge fertility treatments. A decade ago, he pioneered mitochondrial replacement therapy, paving the way for the world's first "three-parent" babies to be born free of a devastating inherited disease.
He sees his work toward embryo gene therapy as not only moral, but necessary.
In 2017, he shocked the world again when his group at Oregon Health and Science University became the first to repair a genetic mutation causing heart disease in dozens of human embryos. The embryos were later destroyed a part of the experiment; current policy in the U.S. prohibits such research from moving into clinical trials.
And that policy doesn't look like it's going to change anytime soon, despite recent political wavering. Last month, a House subcommittee dropped the ban that has blocked the Food and Drug Administration since 2015 from considering any clinical trials of genetically altered embryos intended to create a baby. The move raised the hopes of supporters who want to see such research move forward and angered critics who feel that the science is getting ahead of the ethics. But yesterday, a House committee decided to restore the ban on gene-edited babies after all.
As for Mitalipov, he told leapsmag that he sees his work toward embryo gene therapy as not only moral, but necessary. This interview has been edited and condensed for clarity.
What motivates you to pursue this line of research, even though it is highly controversial?
It's my expertise, I'm an embryologist. We study early development in humans -- sperm, egg, and the first five days of development -- and try to use our knowledge to treat human diseases, particularly in that early stage. This is how IVF started, as a treatment for infertility. It's a very successful cell therapy treatment, with millions of children born. [Now the idea is] to actually to use this IVF platform not as much to treat infertility, but also to treat heritable genetic diseases, because this is a very important stage when gametes from either dad or mom will transmit mutations. This is the bottleneck where we could actually interfere and repair that mutation.
Many people are hesitant to support embryo editing because of "designer babies," yet polls do show that Americans are more open to embryo editing for the purpose of disease prevention. Where should society draw a line?
Yeah, I agree with most Americans that we don't have to edit -- meaning you could make all kind of changes. Instead we do gene repair, which is a therapeutic application.
Gene repair is quite different than gene editing. It involves [focusing on] already known disease-causing mutations and how we can turn them back to normal.
Thousands of gene mutations cause human diseases, like Crohn's, for example, or mutations causing cancer, heart disease. These are well-described, well-studied cause-and-effect diseases and we need to do something about it because otherwise it's impossible to treat once the mutation is already passed to a child.
Early intervention is the best in any disease, but in genetics, "early" means you have to do it at the time of fertilization. That's when we are dealing with one copy of the mutation or maybe two, versus when you have a whole body with billions of cells in solid tissues that we cannot really access and target. So this is the most efficient way of preventing thousands and thousands of genetic diseases. I understand that we have to make sure that it's very safe, of course, and efficient as well. But at the same time, I think this is the future. We have to work toward developing these technologies.
"If we continue banning the research everywhere and not funding it, maybe 100 years will not be enough."
What's your opinion of Dr. He Jiankui and the Chinese CRISPR'ed babies?
This is a case where he was doing gene editing, not gene repair. He hasn't corrected anything, he induced a mutation to normal human genes, hoping that this would somehow confer resistance to HIV, which is still unclear.
I think such straightforward editing is unacceptable specifically for human embryos. He's approach has also never been tested in an animal model. That's why the reaction from the public and scientists was very negative, because this is the case where the doctor does this without any expertise in this area, without knowing probably much about what he is doing, and he acquired it without any oversights, which is troubling. And of course, it negatively affects the legitimate research that is going on in some labs.
What might the future of embryo gene therapy look like?
Hopefully in 10 years from now, thousands and thousands of families that know they carry germline mutations…could go through IVF and we would correct it, and they could have healthy children.
Right now, we have some tools. We cannot correct, but we can select. So what happens is the parents become pregnant and then at about three months along, we can biopsy the amniotic fluid and say, "Hey unfortunately you passed on this mutation." And that means this child, if it's born, will be affected, so we give parents a choice of terminating the pregnancy.
Or we could do it much earlier, so parents go to the IVF clinic where we retrieve about ten eggs, after stimulating a woman's ovaries. Each of them will be fertilized so we have ten embryos that develop. We have a five-day window where we can keep them in the lab. And we basically reap a few cells, we do a biopsy from each of these ten, and we say, "Hey embryo number 1 and number 4 are not mutant, but the others are."
Then we can take these two and the other eight usually will be thrown away. That's the technology that we have now. Some ethicists argue on religious grounds that we have this selection technology available, so why do we need germline gene therapy [i.e. repairing the disease-causing mutations in an embryo]?
I don't understand the moral argument there, because all the available technology is based on selective destruction of the embryo.
With [IVF gene therapy], we will take ten embryos and every embryo we'll make healthy because we can get rid of the mutations. How could embryo destruction be morally superior?
How long do you think it will take for this technology to be available to prospective parents?
It depends how many legitimate labs with expertise can get into this field and resolve all the scientific questions. If we continue banning the research everywhere and not funding it, maybe 100 years will not be enough.
So far, I think that my lab is the only one legitimately working on it. But we would like five, 10, maybe 100 labs in this country and Europe really working. Because we have scientific challenges that we need to resolve before we could say, "Hey now we know how to correct [a given mutation] and now this could be efficient, and there are no side effects or very little." And then we could say, "Okay, I think we've done everything we could in petri dishes and in animals, and now we are ready to transplant this embryo in a patient and see what happens."
"There's just no way you could sink your head into the sand and say, 'Oh, we just ban it and then hopefully everything will go away.'"
Does banning emerging technology actually work?
Banning it usually means it will leak out to a gray area where there's no regulation and many private IVF clinics will just use it while it is still premature. So I think we have to regulate the clinical testing. There's just no way you could sink your head into the sand and say, "Oh, we just ban it and then hopefully everything will go away." That's not going to happen.
If this technology does become feasible and legal in the future, do you think that more and more couples will choose IVF and gene therapy versus the natural method of rolling the dice?
As sequencing technology is becoming available, like 23andMe, more and more parents will realize what kind of mutations they carry. And if your spouse carries the same mutation on the same locus, now you have very high chance of transmitting it. Most of the time today, we find out these families carry it once they have one or two children with that condition.
Of course, parents can just do it naturally in the bedroom and have a chance of transmitting or not transmitting mutations, but hopefully eventually we can say, "Hey, because of your condition, you don't want to play this Russian Roulette. Let's just do IVF." And hopefully the government will cover that kind of treatment because right now IVF is not covered in most states. And we do this therapy and then they have a healthy child.
We have 10,000 different mutations in the human population. That means probably billions of people carry mutations. And unless they go through this gene therapy through IVF, they will keep transmitting them. And we're going to keep having millions and millions of children with diseases. We have to do something about it.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Can Soil Solve the Climate Crisis?
A child sits on the cracked earth, staring at the sun.
When Rattan Lal was awarded the Japan Prize for Biological Production, Ecology in April—the Asian equivalent of a Nobel—the audience at Tokyo's National Theatre included the emperor and empress. Lal's acceptance speech, however, was down-to-earth in the most literal sense.
Carbon, in its proper place, holds landscapes and ecosystems together.
"I'd like to begin, rather unconventionally, with the conclusion of my presentation," he told the assembled dignitaries. "And the conclusion is four words: In soil we trust."
That statement could serve as the motto for a climate crisis-fighting strategy that has gained remarkable momentum over the past five years or so—and whose rise to international prominence was reflected in that glittering award ceremony. Lal, a septuagenarian professor of soil science at Ohio State University, is one of the foremost exponents of carbon farming, an approach that centers on correcting a man-made, planetary chemical imbalance.
A Solution to Several Problems at Once?
The chemical in question is carbon. Too much of it in the atmosphere (in the form of carbon dioxide, a potent greenhouse gas) is the main driver of global heating. Too little of it in the soil is the bane of farmers in many parts of the world, and a threat to our ability to feed a ballooning global population. Advocates say agriculture can mitigate both problems—by adopting techniques that keep more soil carbon from escaping skyward, and draw more atmospheric carbon down into fields and pastures.
The potential impacts go beyond slowing climate change and boosting food production. "There are so many benefits," says Lal. "Water quality, drought, flooding, biodiversity—this is a natural solution for all these problems." That's because carbon, in its proper place, holds landscapes and ecosystems together. Plants extract it from the air and convert it into sugars for energy; they also transfer it to the soil through their roots and in the process of decomposition. In the ground, carbon feeds microbes and fungi that form the basis of complex food webs. It helps soil absorb and retain water, resist erosion, and hold onto nitrogen and phosphorous—keeping those nutrients from running off into waterways and creating toxic algal blooms.
Government and private support for research into carbon-conscious agriculture is on the rise, and growing numbers of farmers are exploring such methods. How much difference these methods can make, however, remains a matter of debate. Lal sees carbon farming as a way to buy time until CO2 emissions can be brought under control. Skeptics insist that such projections are overly optimistic. Some allies, meanwhile, think Lal's vision is too timid. "Farming can actually fix the climate," says Tim LaSalle, co-founder of the Center for Regenerative Agriculture at California State University, Chico. "That should be our only focus."
Yet Can soil solve the climate crisis? may be not be the key question in assessing the promise of carbon farming, since it implies that action is worthwhile only if a solution is ensured. A more urgent line of inquiry might be: Can the climate crisis be solved without addressing soil?
A Chance Meeting Leads to the Mission of a Lifetime
Lal was among the earliest scientists to grapple with that question. Born in Pakistan, he grew up on a tiny subsistence farm in India, where his family had fled as refugees. The only one of his siblings who learned to read and write, he attended a local agricultural university, then headed to Ohio State on scholarship for his PhD. In 1982, he was working at the International Institute of Tropical Agriculture in Nigeria, trying to develop sustainable alternatives to Africa's traditional slash-and-burn farming, when a distinguished visitor dropped by: oceanographer Roger Revelle, who 25 years earlier had published the first paper warning that fossil fuel combustion could throw the climate dangerously off-kilter.

Rattan Lal, Distinguished University Professor of Soil Science at Ohio State, received the Japan Prize at a ceremony in April.
(Photo: Ken Chamberlain. CFAES.)
Lal showed Revelle the soil in his test plots—hard and reddish, like much of Africa's agricultural land. Then (as described in Kristin Ohlson's book The Soil Will Save Us), he led the visitor to the nearby forest, where the soil was dark, soft, and wriggling with earthworms. In the forest, the soil's carbon content was 2 to 3 percent; in Lal's plots, it had dwindled to 0.5 percent. When Revelle asked him where all that carbon had gone, Lal confessed he didn't know. Revelle suggested that much of it might have floated into the atmosphere, adding to the burden of greenhouse gases. "Since then," Lal told me, "I've been looking for ways to put it back."
Back at Ohio State, Lal found that the United States Department of Agriculture (USDA) and Environmental Protection Agency (EPA) were also interested in the connection between soil carbon and climate change. With a small group of other scientists, he began investigating the dimensions of the problem, and how it might be solved.
Comparing carbon in forested and cultivated soils around the globe, the researchers calculated that about 100 billion tons had vanished into the air since the dawn of agriculture 10,000 years ago. The culprits were common practices—including plowing, overgrazing, and keeping fallow fields bare—that exposed soil carbon to oxygen, transforming it into carbon dioxide. Yet the process could also be reversed, Lal and his colleagues argued. Although there was a limit to the amount of carbon that soil could hold, they theorized that it would be possible to sequester several billion tons of global CO2 emissions each year for decades before reaching maximum capacity.
Lal set up projects on five continents to explore practices that could help accomplish that goal, such as minimizing tillage, planting cover crops, and leaving residue on fields after harvest. He organized conferences, pumped out papers and books. As other researchers launched similar efforts, policymakers worldwide took notice.
But before long, recalls Colorado State University soil scientist Keith Paustian (a fellow carbon-farming pioneer, who served with Lal on the UN's International Panel on Climate Change), official attention "kind of faded away. The bigger imperative was to cut emissions." And because agriculture accounted for only about 13 percent of greenhouse gas pollution, Paustian says, the sectors that emitted the most—energy and transportation—got the bulk of funding.
A Movement on the Rise
In recent years, however, carbon farming has begun to look like an idea whose time has come. One factor is that efforts to reduce emissions haven't worked; in 2018 alone, global CO2 output rose by an estimated 2.7 percent, according to the Global Carbon Project. Last month, researchers from the Scripps Institute of Oceanography reported that atmospheric CO2—under 350 ppm when Lal began his quest—had reached 415 ppm, the highest in 3 million years. And with the world's population expected to approach 10 billion by 2050, the need for sustainable technologies to augment food production has grown increasingly pressing.
Today, carbon-conscious methods are central to the burgeoning movement known as "regenerative agriculture," which also embraces other practices aimed at improving soil health and farming in an ecologically sound (though not always strictly organic) manner. In the United States, the latest Farm Bill includes $25 million to incentivize soil-based carbon sequestration. State and local governments across the country are supporting such efforts, as are at least a dozen nonprofits. The Department of Energy's Advanced Projects Research Agency (ARPA-e) is working to develop crops and technologies aimed at increasing soil carbon accumulation by 50 percent. General Mills recently announced plans to advance regenerative farming on 1 million acres by 2030, and many smaller companies have made their own commitments.
The toughest challenge, Lal suggests, may be persuading farmers to change their ways.
Internationally, the biggest initiative is the French-led "4 per 1,000" initiative, which aims to increase the amount of carbon in the soil of farms and rangelands worldwide by 0.4 percent per year—a rate that the project's website contends would "halt the increase of CO2 (carbon dioxide) concentration in the atmosphere related to human activities."
Given the current pace of research, Lal believes that goal—which equates to sequestering 3.6 billion tons of CO2 annually, or 10 percent of global emissions—is doable. The toughest challenge, he suggests, may be persuading farmers to change their ways. Although carbon farming can reduce costs for chemical inputs such as herbicides and fertilizers, while building rich topsoil, agriculturalists tend to be a conservative lot.
And getting low-income farmers to leave crop residue on fields, instead of using it for fuel or animal feed, will require more than speeches about melting glaciers. Lal proposes a $16 per acre subsidy, totaling $64 billion for the world's 4 billion acres of cropland. "That's not a very large amount," he says, "if you're investing in the health of the planet."
Experimental Methods Attract Supporters and Skeptics
Some experts question whether enough CO2 can be stashed in the soil to prevent the rise in average global temperature from surpassing the 2º C mark—set by the 2016 Paris Agreement as the limit beyond which climate change would become catastrophic. But others insist that carbon farming's goal should be to reverse climate change, not just to put it on pause.
"That's the only way out of this predicament," says Tim LaSalle, whose Center for Regenerative Agriculture supports the use of experimental methods ranging from multi-species cover cropping to fungal-dominant compost solutions. Using such techniques, a few researchers and farmers claim to be able to transfer carbon to the soil at rates many times higher than with established practices. Although several of these methods have yet to be documented in peer-reviewed studies, LaSalle believes they point the way forward. "We can't fix the climate, or even come close to it, using Rattan's numbers," he says, referring to Lal. "If we can replicate these experiments, we can fix it."
Even scientists sympathetic to regenerative ag warn that relying on unproven techniques is risky. "Some of these claims are beyond anything we've seen in agricultural science," says Andrew McGuire, an agronomist at Washington State University. "They could be right, but extraordinary claims require extraordinary evidence."
Still, the assorted methods currently being tested—which also include amending soil with biochar (made by heating agricultural wastes with minimal oxygen), planting long-rooted perennial crops instead of short-rooted annuals, and deploying grazing animals in ways that enrich soil rather than depleting it—offer a catalogue of hope at a time when environmental despair is all too tempting.
Last October, the National Academy of Sciences, Engineering, and Medicine issued a report acknowledging that it was too late to stave off apocalyptic overheating just by reducing CO2 emissions; removing carbon from the atmosphere would be necessary as well. The document laid out several options for doing so—most of which, it cautioned, had serious limitations.
"Soil is a bridge to the future. We can't do without it."
One possibility was planting more forests. To absorb enough carbon dioxide, however, trees might have to replace areas of farmland, reducing the food supply. Another option was creating biomass plantations to fuel power plants, whose emissions would be stored underground. But land use would be a problem: "You'd need to cover an area the size of India," explains Paustian, who was a co-author of the report. Yet another alternative was direct-air capture, in which chemical processes would be used to extract CO2 from the air. The technology was still in its infancy, though—and the costs and power requirements would likely be astronomical.
The report took up agriculture-based methods on page 95. Those needed further research as well, the authors wrote, to determine which approaches would be most effective. But of all the alternatives, this one seemed the least problematic. "Soil carbon is probably what you can do first, cheapest, and with the most additional co-benefits," says Paustian. "If we can make progress in that area, it's a huge advantage."
In any case, he and other researchers agree, we have little choice but to try. "Soil is a bridge to the future," Lal says. "We can't do without it."