Scientists Are Growing an Edible Cholera Vaccine in Rice

The world's attention has been focused on the coronavirus crisis but Yemen, Bangladesh and many others countries in Asia and Africa are also in the grips of another pandemic: cholera. The current cholera pandemic first emerged in the 1970s and has devastated many communities in low-income countries. Each year, cholera is responsible for an estimated 1.3 million to 4 million cases and 21,000 to 143,000 deaths worldwide.
Immunologist Hiroshi Kiyono and his team at the University of Tokyo hope they can be part of the solution: They're making a cholera vaccine out of rice.
"It is much less expensive than a traditional vaccine, by a long shot."
Cholera is caused by eating food or drinking water that's contaminated by the feces of a person infected with the cholera bacteria, Vibrio cholerae. The bacteria produces the cholera toxin in the intestines, leading to vomiting, diarrhea and severe dehydration. Cholera can kill within hours of infection if it if's not treated quickly.
Current cholera vaccines are mainly oral. The most common oral are given in two doses and are made out of animal or insect cells that are infected with killed or weakened cholera bacteria. Dukoral also includes cells infected with CTB, a non-harmful part of the cholera toxin. Scientists grow cells containing the cholera bacteria and the CTB in bioreactors, large tanks in which conditions can be carefully controlled.
These cholera vaccines offer moderate protection but it wears off relatively quickly. Cold storage can also be an issue. The most common oral vaccines can be stored at room temperature but only for 14 days.
"Current vaccines confer around 60% efficacy over five years post-vaccination," says Lucy Breakwell, who leads the U.S. Centers for Disease Control and Prevention's cholera work within Global Immunization Division. Given the limited protection, refrigeration issue, and the fact that current oral vaccines require two disease, delivery of cholera vaccines in a campaign or emergency setting can be challenging. "There is a need to develop and test new vaccines to improve public health response to cholera outbreaks."
A New Kind of Vaccine
Kiyono and scientists at Tokyo University are creating a new, plant-based cholera vaccine dubbed MucoRice-CTB. The researchers genetically modify rice so that it contains CTB, a non-harmful part of the cholera toxin. The rice is crushed into a powder, mixed with saline solution and then drunk. The digestive tract is lined with mucosal membranes which contain the mucosal immune system. The mucosal immune system gets trained to recognize the cholera toxin as the rice passes through the intestines.
The cholera toxin has two main parts: the A subunit, which is harmful, and the B subunit, also known as CTB, which is nontoxic but allows the cholera bacteria to attach to gut cells. By inducing CTB-specific antibodies, "we might be able to block the binding of the vaccine toxin to gut cells, leading to the prevention of the toxin causing diarrhea," Kiyono says.
Kiyono studies the immune responses that occur at mucosal membranes across the body. He chose to focus on cholera because he wanted to replicate the way traditional vaccines work to get mucosal membranes in the digestive tract to produce an immune response. The difference is that his team is creating a food-based vaccine to induce this immune response. They are also solely focusing on getting the vaccine to induce antibodies for the cholera toxin. Since the cholera toxin is responsible for bacteria sticking to gut cells, the hope is that they can stop this process by producing antibodies for the cholera toxin. Current cholera vaccines target the cholera bacteria or both the bacteria and the toxin.
David Pascual, an expert in infectious diseases and immunology at the University of Florida, thinks that the MucoRice vaccine has huge promise. "I truly believe that the development of a food-based vaccine can be effective. CTB has a natural affinity for sampling cells in the gut to adhere, be processed, and then stimulate our immune system, he says. "In addition to vaccinating the gut, MucoRice has the potential to touch other mucosal surfaces in the mouth, which can help generate an immune response locally in the mouth and distally in the gut."
Cost Effectiveness
Kiyono says the MucoRice vaccine is much cheaper to produce than a traditional vaccine. Current vaccines need expensive bioreactors to grow cell cultures under very controlled, sterile conditions. This makes them expensive to manufacture, as different types of cell cultures need to be grown in separate buildings to avoid any chance of contamination. MucoRice doesn't require such an expensive manufacturing process because the rice plants themselves act as bioreactors.
The MucoRice vaccine also doesn't require the high cost of cold storage. It can be stored at room temperature for up to three years unlike traditional vaccines. "Plant-based vaccine development platforms present an exciting tool to reduce vaccine manufacturing costs, expand vaccine shelf life, and remove refrigeration requirements, all of which are factors that can limit vaccine supply and accessibility," Breakwell says.
Kathleen Hefferon, a microbiologist at Cornell University agrees. "It is much less expensive than a traditional vaccine, by a long shot," she says. "The fact that it is made in rice means the vaccine can be stored for long periods on the shelf, without losing its activity."
A plant-based vaccine may even be able to address vaccine hesitancy, which has become a growing problem in recent years. Hefferon suggests that "using well-known food plants may serve to reduce the anxiety of some vaccine hesitant people."
Challenges of Plant Vaccines
Despite their advantages, no plant-based vaccines have been commercialized for human use. There are a number of reasons for this, ranging from the potential for too much variation in plants to the lack of facilities large enough to grow crops that comply with good manufacturing practices. Several plant vaccines for diseases like HIV and COVID-19 are in development, but they're still in early stages.
In developing the MucoRice vaccine, scientists at the University of Tokyo have tried to overcome some of the problems with plant vaccines. They've created a closed facility where they can grow rice plants directly in nutrient-rich water rather than soil. This ensures they can grow crops all year round in a space that satisfies regulations. There's also less chance for variation since the environment is tightly controlled.
Clinical Trials and Beyond
After successfully growing rice plants containing the vaccine, the team carried out their first clinical trial. It was completed early this year. Thirty participants received a placebo and 30 received the vaccine. They were all Japanese men between the ages of 20 and 40 years old. 60 percent produced antibodies against the cholera toxin with no side effects. It was a promising result. However, there are still some issues Kiyono's team need to address.
The vaccine may not provide enough protection on its own. The antigen in any vaccine is the substance it contains to induce an immune response. For the MucoRice vaccine, the antigen is not the cholera bacteria itself but the cholera toxin the bacteria produces.
"The development of the antigen in rice is innovative," says David Sack, a professor at John Hopkins University and expert in cholera vaccine development. "But antibodies against only the toxin have not been very protective. The major protective antigen is thought to be the LPS." LPS, or lipopolysaccharide, is a component of the outer wall of the cholera bacteria that plays an important role in eliciting an immune response.
The Japanese team is considering getting the rice to also express the O antigen, a core part of the LPS. Further investigation and clinical trials will look into improving the vaccine's efficacy.
Beyond cholera, Kiyono hopes that the vaccine platform could one day be used to make cost-effective vaccines for other pathogens, such as norovirus or coronavirus.
"We believe the MucoRice system may become a new generation of vaccine production, storage, and delivery system."
Dr. Paolo Macchiarini, then a professor of Regenerative Medicine at Karolinska Institute in Stockholm (Sweden), looks on during a plenary session at the Science of the Future international scientific conference, on September 17, 2014.
[Editor's Note: This is the first comprehensive account of the whistleblowers' side of a scandal that rocked the most hallowed halls in science – the same establishment that just last week awarded the Nobel Prize in Medicine. This still-unfolding saga is a cautionary tale about corruption, hype, and power that raises profound questions about how to uphold integrity in scientific research.]
When the world-famous Karolinska Institutet (KI) in Stockholm hired Dr. Paolo Macchiarini, he was considered a star surgeon and groundbreaking stem cell researcher. Handsome, charming and charismatic, Macchiarini was known as a trailblazer in a field that holds hope for curing a vast array of diseases.
It appeared that Macchiarini's miracle cure was working just as expected.
He claimed that he was regenerating human windpipes by seeding plastic scaffolds with stem cells from the patient's own bone marrow—a holy grail in medicine because the body will not reject its own cells. For patients who had trouble breathing due to advanced illness, a trachea made of their own cells would be a game-changer. Supposedly, the bone marrow cells repopulated the synthetic scaffolds with functioning, mucus-secreting epithelial cells, creating a new trachea that would become integrated into the patient's respiratory system as a living, breathing part. Macchiarini said as much in a dazzling presentation to his new colleagues at Karolinska, which is home to the Nobel Assembly – the body that has awarded the Nobel Prizes in Physiology or Medicine since 1901.
Karl-Henrik Grinnemo was a young cardiothoracic surgeon and researcher at Karolinska in 2010, when Macchiarini was hired. "He gave a fantastic presentation with lots of animation and everyone was impressed," Grinnemo says of his first encounter with Macchiarini. Grinnemo's own work focused on heart and aortic valve regeneration, also in the field of stem cell research. He and his colleagues were to help establish an interdisciplinary umbrella organization, under Macchiarini's leadership, called the Advanced Center for Translational Regenerative Medicine, which would aim to deliver cures from Karolinska's world-class laboratories to the bedsides of patients in desperate need.
Little did Grinnemo know that when KI hired Macchiarini, they had ignored a warning that the star surgeon had been accused of scientific misconduct by a colleague who had worked with him at the University of Florence. That blind eye would eventually cost three patients their lives in Sweden.
"A MIRACLE CURE"?
It has been said that if all you have is a hammer, everything looks like a nail, and it wasn't long before Macchiarini announced that he had a patient in dire need of one of the new artificial tracheas. The patient, a native of Eritrea who had emigrated to Iceland, had a slowly growing tumor on his trachea. Macchiarini had previously generated new windpipes from human donor tracheas outside of Sweden, but the Icelandic patient was the first to receive a synthetic trachea implant at Karolinska University Hospital. Macchiarini had already performed a similar procedure with decellularized donor tracheas on other patients around Europe, but not much was known at the time about their outcomes.
Of course, to justify a radical procedure such as removing a patient's trachea, one would need compelling evidence of effectiveness in animal studies, as well as an exhaustion of all other treatment alternatives. Macchiarini claimed that both conditions were met. He performed the implantation of the synthetic trachea as if he had received a hospital exemption. This is comparable to what the U.S. Food and Drug Administration classifies as "compassionate use," a procedure performed only in extreme circumstances, usually when the patient is terminal, and when no available alternative has worked.
Macchiarini personally invited Grinnemo to watch the all-day surgery, and, once the transplant was done after 10 grueling hours, Macchiarini asked him to close the patient. Then the 36-year-old man was transferred to another hospital, where Grinnemo and other attending physicians had little opportunity to follow his long-term recovery.
Two months later, Macchiarini approached Grinnemo with an invitation to be one of multiple co-authors on a paper about the case targeted for the New England Journal of Medicine. This was a huge opportunity for a junior researcher, and Grinnemo gladly agreed to write a one-month follow-up report on the Icelandic patient's clinical condition. He consulted the patient's medical records, which described a man with an infection in one lung but otherwise doing well, and wrote up his contribution. The patient had already been transferred back to Iceland by then and was home from the hospital. It appeared that Macchiarini's miracle cure was working just as expected.
But the ground was beginning to shake.
"We cannot find one word of evidence that points to regeneration induced by stem cells."
On September 2, 2011, three months after the Icelandic patient's surgery, a professor in Leuven, Belgium sent a written warning to KI's vice chancellor, Harriett Wallberg-Henriksson, stating that Macchiarini was guilty of prior research misconduct. This letter was forwarded to the new president at KI, professor Anders Hamsten, urging him to put a halt to more synthetic trachea implants. The accusations were grave.
Professor Pierre Delaere at Kathiolieke Universiteit asserted that synthetic tracheas coated with bone marrow cells did not, as Macchiarini had claimed, transform into living tracheas. He cited "countless" failures in animal experiments and called the outcome of Macchiarini's previous human surgeries "disastrous…half the patients died. The others are in a palliative setting….We cannot find one word of evidence that points to regeneration induced by stem cells."
Once again, KI simply ignored the warning, and Grinnemo and the 24 co-authors on the splashy academic paper about the latest surgery didn't even know about it. In the meantime, the New England Journal of Medicine rejected it for lacking a longer follow-up on the patients and missing data on how well the implants had integrated with the patient's respiratory system, so Macchiarini submitted it to The Lancet instead.
And he kept performing his experimental surgeries.
Soon there was a second transplant patient, a 30-year-old American man named Christopher Lyles. After his operation at KI, he returned to the U.S and the Swedish doctors were unable to follow his progress. Three months after his surgery, they learned that he had died at his home.
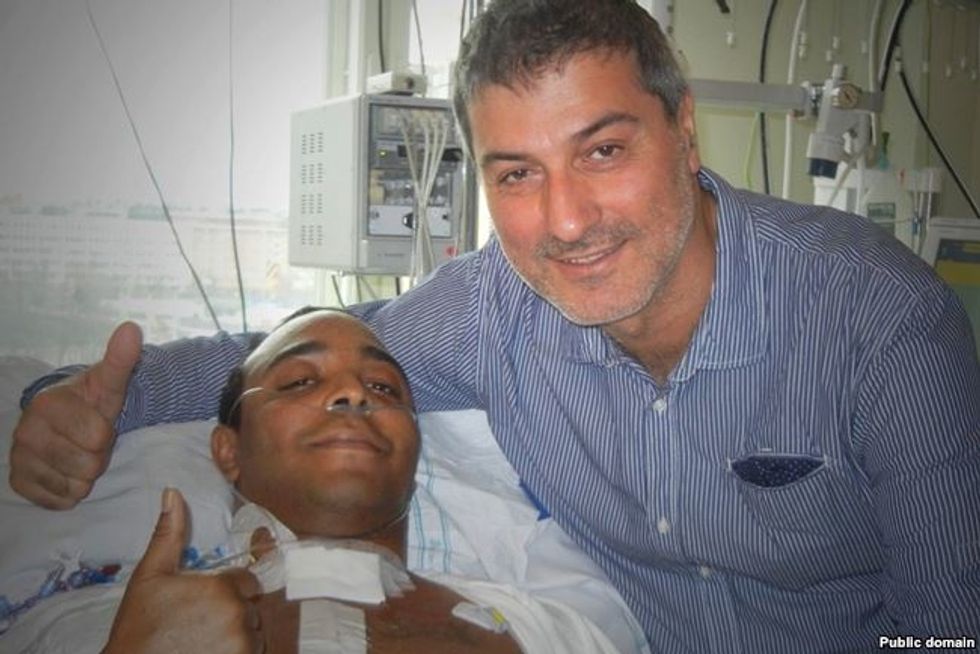
Paolo Macchiarini with Christopher Lyles, the American patient on whom he performed a trachea transplant in Stockholm in 2011. Lyles died a few months later.
Only four months after Lyles died, the third patient, a 22-year-old Turkish woman, received one of Macchiarini's grafts. In all three patients, Macchiarini had claimed that they were in dire straights—terminal if not for the hope of a trachea transplant, and he claimed a hospital exemption in all three cases. In fact, Grinnemo says, all three had been in stable condition before their surgeries—a reality Macchiarini did not share with his collaborators and co-authors on two academic papers about the surgeries that were subsequently published in The Lancet.
The Turkish woman's story is especially tragic. The young woman had initially undergone surgery elsewhere to fix an unrelated problem—hand sweating--but wound up with an accidentally damaged trachea that set her on a course of utter devastation. She sought help from Macchiarini, but his graft operation left her "living in hell," says Grinnemo. In intensive care afterward, her airways were producing so much mucus that they had to be cleared every four hours around the clock. The procedure "is like someone keeping your head under water every fourth hour until you almost suffocate to death. This is something that you wouldn't wish on your worst enemy," says Grinnemo.
By the spring of 2013, six months after Macchiarini's operation, the graft began to collapse. Several metal stents were inserted into her airways, but each one only worked for a short while. Macchiarini decided to remove the first plastic trachea and implant a new one. It seemed she couldn't get any worse, but after the second transplant, the young woman further deteriorated. Her airway secretions only increased; she had to undergo thousands of bronchoscopies, where an instrument was pushed down her throat into her lungs, and hundreds of surgeries during her three-year stint in the intensive care unit. Her body couldn't tolerate much more.
The whistleblowers realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Grinnemo, together with fellow KI physicians Matthias Corbascio, Oscar Simonson and Thomas Fux, who were all involved in the care of the Turkish woman, became alarmed when the Icelandic patient came back to their hospital in the fall of 2013 with similar complaints. They realized that, despite Macchiarini's claims of successful operations in several now-published papers, the patients had been mutilated.
Both the Icelandic patient and the Turkish woman were too incapacitated to speak for themselves, so in the late fall of 2013, Grinnemo and his three concerned colleagues reached out to the patients' relatives seeking permission to review their medical records. It took weeks to receive the permissions, but once they did, what they found stunned them.
The Icelandic patient had developed fistulas (holes) between the artificial trachea and his esophagus, and had been fitted with several stents. Soon his esophagus also had to be removed, which Macchiarini was aware of. He should have reported these complications in the articles on which he was lead author, Grinnemo contends, and also should have informed his co-authors, each of whom had been responsible for writing up discrete sections of the papers. But Macchiarini had described each transplant as a success and had greatly exaggerated, if not outright lied, about how each patient had fared.
THE WHISTLEBLOWERS FIGHT BACK
Grinnemo and several other suspicious colleagues decided to launch an investigation. The result was a 500-page report identifying the synthetic tracheas as the problem and revealing that Macchiarini had falsified data and suppressed critical information in his reporting. He had even invented biopsies of the grafts, claiming that the marrow cells had populated them with functioning epithelial cells, while there was no real evidence of the patients' cells growing to line the tracheas.
The whistleblowers also discovered that Macchiarini had never received ethical clearance from Sweden's Human Ethical Review Board, nor had he gotten approval for his plastic tracheas from the Medical Product Agency, the Swedish counterpart to the FDA. He had relied entirely on his ability to do the surgeries under the hospital exemption, which he made everyone believe that he had obtained thanks to his star power.
What Macchiarini was doing, the investigators realized, was experimentation on living human subjects; he had circumvented the normal oversight protocols that exist to protect such subjects.
At a procedural meeting with his colleagues, including Dr. Ulf Lockowandt, the head of Karolinska University Hospital's Department of Cardiothoracic Surgery, Macchiarini dismissed the patients' complications as "manageable."
But among the large interdisciplinary team whose members had knowledge only of their own discrete specialties, doubts about Macchiarini's technique were festering. Complications in the patients only worsened when the tracheas inevitably began to collapse. There was a bursting open of sutures, holes in tissues adjacent to the implants, the disintegration of tissues that clogged bronchial passages. In far more than half of all the patients Macchiarini had operated on in several countries, patients died a lingering and agonizing death.
The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
When Grinnemo and his fellow investigators dug all this up, they decided they had to report it to the very top of Karolinska, to the institute's president, Anders Hamsten, so that he could stop Macchiarini from performing any further transplants. The last thing the whistleblowers expected was for the full weight of the institution to come crashing down against them.
"THEY WANTED TO SILENCE EVERYTHING"
KI had ample reason to sweep criticisms of Macchiarini under the rug. Up to 100 patients were about to be recruited for an international clinical study in which Macchiarini would do his implants—a nightmarish prospect considering his track record. But KI stood to receive millions of dollars in a government grant to conduct the study across Europe and Russia.
Still other incentives existed for KI to suppress Macchiarini's record. Plans were underway to establish a stem cell center in Hong Kong with over $45 million provided by a wealthy Chinese businessman. At the center, Macchiarini would be able to do his trachea transplants on patients in Asia. And in addition to the financial incentives to keep Macchiarini's brand associated with KI, many high-powered individuals were involved in his initial recruitment and didn't want their reputations tarnished, Grinnemo says. KI not only ignored the whistleblowers' allegations; punishment against them was swift and decisive.
On March 7, 2014, Grinnemo and the other whistleblowers met with Dr. Hamsten, in addition to two of Macchiarini's supervisors and the director of KI's Regenerative Network. They presented their findings and requested an official investigation by KI, including scrutiny of the now-six published research papers in which Macchiarini had claimed the success of his implants in humans. The whistleblowers also told the leadership about some rat studies Macchiarini had published in a prestigious journal that appeared to rely on falsified data.
Instead of the welcoming reception they expected, the room bristled with hostility. "I basically forced them to agree to an investigation," Grinnemo says, "but it was a very tough meeting. The feeling I got was that they wanted to silence everything and that they would continue to silence me and the other whistleblowers. We were already feeling the backlash."

From the left, whistleblowers Matthias Corbascio, Oscar Simonson, Thomas Fux and Karl-Henrik Grinnemo.
Previously, Grinnemo had confronted Macchiarini with questions about patients he had implanted in Russia prior to his stint at Karolinska. "Paolo Macchiarini realized we were onto something and he became very angry. He said he would do everything in his power to make my life miserable," Grinnemo recalls.
Macchiarini made good on his threat by filing a complaint about Grinnemo with the Swedish Research Council, the main funder of research in Sweden. At the time, Macchiarini and Grinnemo had jointly submitted a grant application on an aortic valve regeneration project, which the Council had approved. Macchiarini suddenly complained that Grinnemo had stolen his data on aortic valve regeneration, even though, unlike Grinnemo, Macchiarini was not a heart surgeon and had conducted no research on heart structures. In reality, all of the data had been generated by Grinnemo. The Council did a review and concluded that Grinnemo had not stolen the data, but Macchiarini spread rumors throughout KI that the young researcher was guilty of scientific misconduct. "He wanted to discredit me because he knew I was dangerous and he wanted to stop anyone from believing me," Grinnemo says.
In spite of the findings from the Council that he had committed no scientific misconduct, KI opened an investigation—not of Macchiarini, but of Grinnemo himself. It soon became clear that KI also wanted to discredit Grinnemo and to silence any possible rumors about Macchiarini's conduct. The whistleblowers continued to push forward, however, and over a period of several weeks they wrote to president Hamsten four times, asking that KI investigate the deadly transplants still being promoted by Macchiarini as some kind of miracle cure.
After four written requests, Hamsten replied that if the whistleblowers had concerns about Macchiarini, they should contact their supervisors or write a formal complaint. But the whistleblowers had already contacted several individuals in supervisory roles who had made it clear that they wanted nothing to do with the affair. It was obvious that KI would resist any investigation of Macchiarini and that no one, outside of the whistleblowers, wanted to take any responsibility for what could amount to a major scandal at one of the world's most powerful academic institutions.
The whistleblowers had another hostile and unproductive meeting with several doctors at KI with whom they shared a letter they had written to the journal Nature Communications, which published Macchiarini's article on rat experimentation, urging them to investigate whether he had falsified the data. Once again, the whistleblowers met with a wall of resistance. Grinnemo was now discredited because of the aortic valve grant application, the doctors reminded him, and no investigation or retraction of the Nature Communications article would be pursued.
In June 2014, KI made its retaliation against Grinnemo official by putting its legal counsel in charge of its investigation of his grant application. The university's ethical board then concluded that Grinnemo should have informed Macchiarini more clearly that he submitted the application to the Swedish Research Council and that he should have obtained a written acceptance from Macchiarini before proceeding with the application. KI could not find Grinnemo guilty of research misconduct, but accused him of "carelessness" regarding the usage of data—which was his own data all along.
A few years later, Grinnemo was totally cleared by both the Central Ethical Review Board and KI. However, the rumors surrounding the investigation and the finding that he hadn't "used data correctly" in a grant application had done their damage to his reputation. Since then, he has not received a single research grant. "You can't appeal the findings," Grinnemo says. "I don't know if I will ever get more research money. I'm totally dead."
The whistleblowers made multiple appeals to Dr. Lockowandt, the head of the Department of Cardiothoracic surgery, for an investigation into Macchiarini's implants, but they were stonewalled from the beginning. Lockowandt did nothing.
"The heads of departments at the KUH and KI didn't actually have that much power," Grinnemo explains. "Dr. Lockowandt thought he was fighting for his own career and position. He's basically a good person who decided to go the route of an administrator, and if you have conflicts with your superiors, your career will be over." In other words, a real investigation of Macchiarini's record could not happen with so much money and prestige riding on the continued presence of the star surgeon.
By August 11, 2014, the whistleblowers had made repeated requests of Dr. Hamsten for a meeting to present the data inconsistencies between Macchiarini's patients' medical records and what he had reported in numerous articles, all published in prestigious medical journals. When they finally received the answer—a cold instruction to submit a written notification to the heads of their departments—it was clear that KI was giving them the runaround.
But rather than simply ignore the whistleblowers, KI apparently decided to double down, trying to discredit them in an intimidation campaign.
KI even went so far as to force the chief medical officer of Karolinska University Hospital, Johan Bratt, to report the whistleblowers to Swedish police, claiming that they violated the law and the patients' privacy when they went through the patients´ charts and submitted their appeals for investigation to KI and the Central Ethical Review Board. KI claimed that their report revealed the identities of patients, even though they had been careful to anonymize all the information. The police interrogated several of the whistleblowers and concluded that they had done no wrong, but the incident made it clear how low KI would sink in its desire to harass them.
"You can't appeal the findings. I don't know if I will ever get more research money. I'm totally dead."
In private, Grinnemo's colleagues supported him, but feared coming forward out of the fear of losing their jobs. Grinnemo himself was in a tough spot. "I knew it would be difficult for me to do research but I hoped my position as a surgeon was secure," he says. "But after the New York Times article, I realized even that position was not as safe as I had thought."
THE MEDIA CATCHES ON -- WITH A PRICE
On November 24, 2014, The New York Times published a front-page story about Paolo Macchiarini based on the whistleblowers' investigation, which had leaked to the press. Officials at KI suspected one or more of the whistleblowers of being the leakers, but the publicity forced the top brass to at least appear to act. The next day they asked Dr. Bengt Gerdin, a professor of surgery at Sweden's Uppsala University, to do an investigation of Dr. Macchiarini. It's hard not to conclude that, after months of stonewalling on an institutional investigation, the Times article compelled them to do something. But KI still did not take any of the pressure off of Grinnemo and his three fellow whistleblowers.
One by one, each was informed that he would receive a formal warning from Dr. Lockowandt, the head of the cardiothoracic clinic, alleging that they had violated patient privacy by reading medical records. The whistleblowers countered that they had informed consents. They also asked for a meeting with Lockowandt and KI's attorneys, to which they brought a union representative and someone from the Swedish version of the American Medical Association. The union representative informed KI's attorneys that the doctors were actually required by law to consult a patient's medical records when the patient's life is in danger. Not doing so would have been a crime. Karolinska backed off on the formal warnings (which would have been the last step before actual termination) after that. But they found other ways to retaliate.
One whistleblower, Oscar Simonson, had been offered a residency at Karolinska University Hospital, but that offer was withdrawn without explanation. Grinnemo had expected to receive an advisor position in cardiothoracic surgery, but that promotion also evaporated. In addition, the number of surgeries he was tapped to perform was reduced and he was relegated to doing the "less popular" standard heart surgeries that began late in the afternoon and evenings.
The grinding day-to-day pressure on the whistleblowers never let up. On December 19, 2014, Dr. Lockowandt informed all four that they had been on the verge of being fired, but that hospital attorneys changed their minds at the last minute. By then not only were their reputations in tatters, but they had invested an estimated 10,000 hours of labor investigating Macchiarini's misconduct, appealing to KI, and defending themselves against KI's harassment.
When interviewed for this article, Grinnemo said, "I have never had a single day of vacation from this situation. In addition to dealing with it, I've been doing surgery and taking care of patients. I've had trouble sleeping, and it has affected my family. I haven't been able to focus on my family, and I feel guilty toward my kids." Of all the whistleblowers, Grinnemo seems to have received the brunt of the backlash.
KI was finally pushed to further action by yet more negative coverage of the Macchiarini affair in the media. In January 2015, Swedish National Television aired an exposé covering the Macchiarini surgeries and the desperate plight of the patients. In response, the Swedish public demanded that KI make a course correction. On February 19, KI withdrew all of its threats of formal warnings to the whistleblowers.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend.
However, progress was incremental. On April 16, KI's ethical committee, which had done its own investigation, acquitted Macchiarini of allegations of scientific misconduct. This is the same university ethical board that had reprimanded Grinnemo over his usage of data in the aortic valve grant application.
The whistleblowers maintain that throughout the summer of 2015, KI was still far more focused on covering up the Macchiarini affair than on getting to the bottom of it. On May 13, the professor from Uppsala submitted the results of his independent investigation, in which he concluded that seven out of seven published articles in which Macchiarini was the lead author entailed the fabrication of data.
KI ignored the report. In August 2015, KI's president announced that Macchiarini had been cleared of all charges of scientific misconduct and that, magically, ethical approvals existed for the patient from Iceland. Macchiarini got a reprimand for being "a little sloppy" in his published descriptions of his patients. Then KI, eager to placate the public and salvage its reputation, held a press conference to announce the presumed innocence of its star surgeon.
As the press event began, KI called the heads of the whistleblowers' departments to tell them to make sure the four didn't attend, according to Grinnemo.
"They seemed to think we would come crashing in to the press conference and make a scene. It's ridiculous, but that's what they thought," says Grinnemo.
Around this time, KI asked that the whistleblowers compile and forward all of their correspondence with the independent investigator on the grounds that they were suspected of manipulating his investigation. The accusation went nowhere; the whistleblowers had barely spoken with him.
Then came a request from KI's IT department for the whistleblowers to compile and submit all of their emails for the preceding year. They were simply told that "an anonymous person" had made the request.
Throughout 2015, KI continued to go after the whistleblowers aggressively. That August, they were so discouraged that they felt they would never obtain any additional grants from the Swedish Research Council or any other funding organizations, and that their academic careers were over. To add insult to injury, a Swedish newspaper published an article defending Macchiarini and concluding that he was not guilty of violating the Helsinki Declaration, a statute put into effect after World War II protecting all humans from unauthorized medical experimentation.
THE TIDE TURNS, BUT REDEMPTION IS ELUSIVE
Then in November, they received a request from a Swedish filmmaker to be interviewed about the Macchiarini affair. Not knowing what angle the film was expected to take, they each put in hours in front of the camera. They wouldn't know the results of their interviews until January 2016, when the three-part documentary, "The Experiments," aired on Swedish television. The film documented the tortuous death of a Russian woman and the suffering of other patients who had received Macchiarini's implants.
That same month, a devastating article on Paolo Macchiarini was published in the American magazine Vanity Fair. Titled "The Celebrity Surgeon Who Used Love, Money and the Pope to Scam an NBC News Producer," the article revealed Macchiarini as an even more prolific fabulist and liar than anyone had remotely suspected. Not only did he fabricate data for multiple scientific papers, he had also lied about everything from his alleged medical training and celebrity connections to his personal relationship status.
Ironically, the woman who ultimately dismantled Macchiarini was Benita Alexander, a former producer for NBC News who was at one point engaged to marry him in a lavish ceremony that Macchiarini promised would be officiated by Pope Francis. Except that he didn't know the Pope, and he was already married to one woman and living with another.
Her story of heartbreak infuriated the public. The full list of people who had believed Macchiarini's almost countless fabrications may never be known—a tribute to his considerable personal charisma. But after the "The Experiments" and the Vanity Fair article, the public had had enough of Paolo Macchiarini. They demanded that KI's president step down and that Macchiarini be fired.

TV producer Benita Alexander appeared as a guest on Dr. Oz's show on February 14th, 2018 to discuss Dr. Macchiarini's deception. "He railroaded my life," she said.
In February 2016, there was a cascade of resignations and firings at KI. First, president Anders Hamsten stepped down. Then several top KI officials, including the General Secretary of the Nobel Assembly, the Dean of Research, and an advisor to KI's president, were either fired or stepped down. On March 3, several members of the board were replaced. The whistleblowers received an award for coming forward by an organization called Transparency International, but instead of heaving a sigh of relief, they only felt a continued sense of foreboding.
"We all felt very vulnerable because we knew that KI would retaliate in some way," says Grinnemo. A fellow whistleblower, Dr. Corbascio, gave an interview on a prime time news program saying that KI was a corrupt institution and should apologize to the patients' families and even pay them for their suffering. After that, both he and another colleague came under intensified scrutiny at work. They say that their supervisors, who were deeply involved in collaborations with Macchiarini, watched everything they did, apparently looking for a reason to fire them.
Grinnemo and Simonson both left KI to work for Uppsala University. But the lasting effects of the scandal followed them there. They still couldn't obtain any grants for new research, and other scientists at KI and elsewhere were unwilling to collaborate with them for fear of their own work being "tainted" by association.
On March 23, 2016, Paolo Macchiarini was finally sacked by KI. Still, the whistleblowers couldn't claim victory.
"Our aim," says Grinnemo, "was not to get him sacked but to stop the grafts, and we knew he would continue to do them in other countries. The clinical trial aiming to recruit 100 or so patients hadn't been halted. We tried to warn the Russian authorities and the EU grant office, and wanted them to stop the grant to Macchiarini. There was no response, so at that time we didn't know if the clinical trial would go forward."
Still, there was reason to hope. News of Macchiarini's scientific fraud, not to mention his personal debacle with Benita Alexander, had made its way around scientific circles in Germany and Britain, where a new investigation began.
Eventually, the entire board at Karolinska was replaced. Under its new president, the institute issued a decree this past summer finding the now thoroughly disgraced Macchiarini guilty of scientific misconduct, and concluding that six of his research papers should be retracted.
But in a cruelly ironic twist, KI took the whistleblowers' own investigation and turned it against them. KI's report found Grinnemo also guilty of scientific misconduct for apparently falling short in the care of the Icelandic patient, even though his role in the case had been minimal. It was like a punch in the gut, because the judgment cast Grinnemo as equally blameworthy to Macchiarini. It also failed to recognize that he had long ago not only withdrawn his name from the offending paper, but lobbied for years to have it retracted.
"This sends the message that whistleblowers in research will be punished. That's a serious problem."
The KI report also established the new category of "blameworthy" to describe two of the whistleblowers for their roles as co-authors in some of the papers. The whistleblowers did not receive a chance to respond to the new accusations before a decision was made to publicly reprimand them.
That decision can't be appealed.
Simonson told Science Magazine, "This sends the message that whistleblowers in research will be punished. That's a serious problem."
These days, Macchiarini is lying low but still publishing his supposed stem cell research, most recently on baboons. A paper published in March of this year in the Journal of Biomedical Materials lists his affiliation as Kazan Federal University in Russia, but in April 2017, the university fired him. He's rumored to be living in Italy and couldn't be reached for this article. He was investigated for criminal activity in Sweden and the case was closed without charges, but Grinnemo says that another prosecutor is now considering whether to bring charges against him for "aggravated manslaughter."
At KI, only Karin Dahlman Wright, who was the Institute's acting president during several months of these events, responded to a request for comment, but she claimed a near-total unawareness of the whistleblowers' narrative. Other officials there declined to be interviewed.
KI's clinical trial that was aiming to recruit new patients for biologically engineered tracheas is no longer happening. The European Commission posted on their research portal that the trial ended on March 31, 2017, stating: "Grant Agreement terminated."
As for Grinnemo, Simonson, Corbascio and Fux, they are still fighting for their careers. Grinnemo is currently suing KI for a chance to defend himself against its accusations of scientific misconduct. He's also claiming damages for lost grant funding, thousands of hours spent defending himself, and harm to his reputation. Whether he will prevail in court remains to be seen.
"KI did a very good job of destroying our careers," says Simonson. "They didn't do anything else well, but they did a very thorough job of that."
This Innovative Startup Is a Lifeline for Patients at Rural Hospitals
Chloe Alpert, the founder of Medinas.
When Jenn Morson Frederick went into labor with her baby in Annapolis, Maryland, she remembers being hooked up intravenously to an infusion pump because she needed antibiotics. She readily admits that the last thing on her mind was what would happen to the pump after she was done with it.
"Ten minutes from where I live, in Oakland, there are children who can't afford care and there are smaller practices just getting eaten up on cost."
In fact, the pump might go on to assist the labor of another new mother at a rural hospital many miles away, thanks to an innovative online marketplace called Medinas Health. Founded last year by a 27-year-old entrepeneur, Medinas Health buys used medical supplies and sells them to under-resourced hospitals who are happy to get functioning equipment at discounted prices.
The startup is built on a machine learning algorithm that uses historical data for medical devices to predict how much longer they can be used and still be sold at optimum prices on the secondary market. This allows hospitals to squeeze the most use out of their supplies.
Such transactions are the lifeblood for rural or critical access hospitals, says Chloe Alpert, the founder and CEO of Medinas. She first came up with the idea when she noticed a glaring discrepancy in the healthcare marketplace: From 2010 to 2016, 79 hospitals had closed their doors and hundreds more were at risk. At the same time, according to the National Academy of Medicine, the United States wastes medical supplies to the tune of $765 billion every year. On a household level, many people are saddled with medical debt: One in six Americans has past due healthcare bills. The numbers shocked Alpert.
What's more, she found that many used medical supplies were being shipped off to developing countries, partly to minimize the hospitals' liability. "[The model was] fundamentally flawed," she says. "I live in San Francisco and ten minutes from where I live, in Oakland, there are children who can't afford care and there are smaller practices just getting eaten up on cost."
Now, through Medinas, hospitals can offload unwanted clinical assets, and other medical offices can buy them at discounted prices. Since its launch in August 2017, the startup has sold just over 100 items, ranging from infusion pumps to an MRI machine.
Typically, hospitals hold onto their medical supplies as long as possible. Proprietary data from Medinas place the life expectancy of something like an infusion pump at ten years.
"Hospitals' biomed departments are going to try to keep that unit going for as long as they can because you have to replace an entire fleet and that's a significant financial overlay," says Suzi Collins, Director of Materials Management at Mountain Vista Medical Center in Gilbert, Ariz.
"I wanted to do something that would actually make an impact. Imagine healthcare costs going down instead of up."
But after many rinse-and-repeat repairs, it might be time to spring for a new unit. Medinas conducts cost-benefit analyses to show whether it's worth the financial cost for a hospital to hold on to old, creaky equipment. In some cases, manufacturers introduce a new version of a pump and discontinue support for older models, forcing hospitals' hands.
That's when Medinas may step in to facilitate the sale of older medical devices to different hospitals, connecting the lives of urban moms like Frederick to rural moms like Kelly Burch, who recently delivered her baby at the Alice Peck Day Memorial Hospital in rural Lebanon, New Hampshire.
At press time, Medinas had recently received more than 700 infusion pumps to sell from an Arizona medical center and was in negotiations with healthcare facilities who might be interested in buying them. For her work with Medinas, Alpert won $500,000 as part of the Forbes 30 Under 30 competition.
"It really blows my mind to see all these inefficiencies in healthcare, to know that Medinas is doing something tangible to address disparities in care," Alpert says. "I wanted to do something that would actually make an impact. Imagine healthcare costs going down instead of up. That is really neat."


