Scientists Are Growing an Edible Cholera Vaccine in Rice

The world's attention has been focused on the coronavirus crisis but Yemen, Bangladesh and many others countries in Asia and Africa are also in the grips of another pandemic: cholera. The current cholera pandemic first emerged in the 1970s and has devastated many communities in low-income countries. Each year, cholera is responsible for an estimated 1.3 million to 4 million cases and 21,000 to 143,000 deaths worldwide.
Immunologist Hiroshi Kiyono and his team at the University of Tokyo hope they can be part of the solution: They're making a cholera vaccine out of rice.
"It is much less expensive than a traditional vaccine, by a long shot."
Cholera is caused by eating food or drinking water that's contaminated by the feces of a person infected with the cholera bacteria, Vibrio cholerae. The bacteria produces the cholera toxin in the intestines, leading to vomiting, diarrhea and severe dehydration. Cholera can kill within hours of infection if it if's not treated quickly.
Current cholera vaccines are mainly oral. The most common oral are given in two doses and are made out of animal or insect cells that are infected with killed or weakened cholera bacteria. Dukoral also includes cells infected with CTB, a non-harmful part of the cholera toxin. Scientists grow cells containing the cholera bacteria and the CTB in bioreactors, large tanks in which conditions can be carefully controlled.
These cholera vaccines offer moderate protection but it wears off relatively quickly. Cold storage can also be an issue. The most common oral vaccines can be stored at room temperature but only for 14 days.
"Current vaccines confer around 60% efficacy over five years post-vaccination," says Lucy Breakwell, who leads the U.S. Centers for Disease Control and Prevention's cholera work within Global Immunization Division. Given the limited protection, refrigeration issue, and the fact that current oral vaccines require two disease, delivery of cholera vaccines in a campaign or emergency setting can be challenging. "There is a need to develop and test new vaccines to improve public health response to cholera outbreaks."
A New Kind of Vaccine
Kiyono and scientists at Tokyo University are creating a new, plant-based cholera vaccine dubbed MucoRice-CTB. The researchers genetically modify rice so that it contains CTB, a non-harmful part of the cholera toxin. The rice is crushed into a powder, mixed with saline solution and then drunk. The digestive tract is lined with mucosal membranes which contain the mucosal immune system. The mucosal immune system gets trained to recognize the cholera toxin as the rice passes through the intestines.
The cholera toxin has two main parts: the A subunit, which is harmful, and the B subunit, also known as CTB, which is nontoxic but allows the cholera bacteria to attach to gut cells. By inducing CTB-specific antibodies, "we might be able to block the binding of the vaccine toxin to gut cells, leading to the prevention of the toxin causing diarrhea," Kiyono says.
Kiyono studies the immune responses that occur at mucosal membranes across the body. He chose to focus on cholera because he wanted to replicate the way traditional vaccines work to get mucosal membranes in the digestive tract to produce an immune response. The difference is that his team is creating a food-based vaccine to induce this immune response. They are also solely focusing on getting the vaccine to induce antibodies for the cholera toxin. Since the cholera toxin is responsible for bacteria sticking to gut cells, the hope is that they can stop this process by producing antibodies for the cholera toxin. Current cholera vaccines target the cholera bacteria or both the bacteria and the toxin.
David Pascual, an expert in infectious diseases and immunology at the University of Florida, thinks that the MucoRice vaccine has huge promise. "I truly believe that the development of a food-based vaccine can be effective. CTB has a natural affinity for sampling cells in the gut to adhere, be processed, and then stimulate our immune system, he says. "In addition to vaccinating the gut, MucoRice has the potential to touch other mucosal surfaces in the mouth, which can help generate an immune response locally in the mouth and distally in the gut."
Cost Effectiveness
Kiyono says the MucoRice vaccine is much cheaper to produce than a traditional vaccine. Current vaccines need expensive bioreactors to grow cell cultures under very controlled, sterile conditions. This makes them expensive to manufacture, as different types of cell cultures need to be grown in separate buildings to avoid any chance of contamination. MucoRice doesn't require such an expensive manufacturing process because the rice plants themselves act as bioreactors.
The MucoRice vaccine also doesn't require the high cost of cold storage. It can be stored at room temperature for up to three years unlike traditional vaccines. "Plant-based vaccine development platforms present an exciting tool to reduce vaccine manufacturing costs, expand vaccine shelf life, and remove refrigeration requirements, all of which are factors that can limit vaccine supply and accessibility," Breakwell says.
Kathleen Hefferon, a microbiologist at Cornell University agrees. "It is much less expensive than a traditional vaccine, by a long shot," she says. "The fact that it is made in rice means the vaccine can be stored for long periods on the shelf, without losing its activity."
A plant-based vaccine may even be able to address vaccine hesitancy, which has become a growing problem in recent years. Hefferon suggests that "using well-known food plants may serve to reduce the anxiety of some vaccine hesitant people."
Challenges of Plant Vaccines
Despite their advantages, no plant-based vaccines have been commercialized for human use. There are a number of reasons for this, ranging from the potential for too much variation in plants to the lack of facilities large enough to grow crops that comply with good manufacturing practices. Several plant vaccines for diseases like HIV and COVID-19 are in development, but they're still in early stages.
In developing the MucoRice vaccine, scientists at the University of Tokyo have tried to overcome some of the problems with plant vaccines. They've created a closed facility where they can grow rice plants directly in nutrient-rich water rather than soil. This ensures they can grow crops all year round in a space that satisfies regulations. There's also less chance for variation since the environment is tightly controlled.
Clinical Trials and Beyond
After successfully growing rice plants containing the vaccine, the team carried out their first clinical trial. It was completed early this year. Thirty participants received a placebo and 30 received the vaccine. They were all Japanese men between the ages of 20 and 40 years old. 60 percent produced antibodies against the cholera toxin with no side effects. It was a promising result. However, there are still some issues Kiyono's team need to address.
The vaccine may not provide enough protection on its own. The antigen in any vaccine is the substance it contains to induce an immune response. For the MucoRice vaccine, the antigen is not the cholera bacteria itself but the cholera toxin the bacteria produces.
"The development of the antigen in rice is innovative," says David Sack, a professor at John Hopkins University and expert in cholera vaccine development. "But antibodies against only the toxin have not been very protective. The major protective antigen is thought to be the LPS." LPS, or lipopolysaccharide, is a component of the outer wall of the cholera bacteria that plays an important role in eliciting an immune response.
The Japanese team is considering getting the rice to also express the O antigen, a core part of the LPS. Further investigation and clinical trials will look into improving the vaccine's efficacy.
Beyond cholera, Kiyono hopes that the vaccine platform could one day be used to make cost-effective vaccines for other pathogens, such as norovirus or coronavirus.
"We believe the MucoRice system may become a new generation of vaccine production, storage, and delivery system."
Electricity is emerging as a powerful treatment for chronic ailments.
Kelly, a case manager for an insurance company, spent years battling both migraines and Crohn's, a disease in which the immune system attacks the intestines.
For many people, like Kelly, a stronger electric boost to the vagus nerve could be life-changing.
After she had her large intestine removed, her body couldn't absorb migraine medication. Last year, about twice a month, she endured migraines so bad she couldn't function. "It would go up to a ten, and I would rock, wait it out," she said. The pain might last for three days.
Then her neurologist showed her a new device, gammaCore, that tames migraines by stimulating a nerve—not medication. "I don't have to put a chemical in my body," she said. "I was thrilled."
At first, Kelly used the device at the onset of a migraine, applying electricity to her pulse at the front of her neck for six minutes. The pain peaked at about half the usual intensity--low enough, she said, that she could go to work. Four months ago, she began using the device for two minutes each night as prevention, and she hasn't had a serious migraine since.
The Department of Defense and Veterans Administration now offer gammaCore to patients, but it hasn't yet been approved by Medicare, Medicaid, or most insurers. A month of therapy costs $600 before insurance or a generous financial assistance program kicks in.

A patient uses gammaCore, a non invasive vagal nerve stimulator device that was FDA approved in November 2018, to treat her migraine.
(Photo captured from a patient video at gammacore.com)
If the poet Walt Whitman wrote "I Sing The Body Electric" today, he might get specific and point to the vagus nerve, a bundle of fibers that run from the brainstem down the neck to the heart and gut. Singing stimulates it—and for many people, like Kelly, a stronger electric boost to the nerve could be life-changing.
The mind-body connection isn't just an idea — the vagus nerve literally carries signals from the mind to the body and back. It may explain the link between childhood trauma and illnesses such as chronic pain and headaches in adults. "How is it possible that a psychological event causes pain years later?" asked Peter Staats, co-founder of electroCore, which has won approval for its new device from the Food and Drug Administration (FDA) for both migraine and cluster headaches. "There has to be a mind-body interface, and that is the vagus nerve," he said.
Scientists knew that this nerve controlled your heart rate and blood pressure, but in the past decade it has been linked to both pain and the immune system.
"Everything is gated through the vagus -- problems with the gut, the heart, and the lungs," said Chris Wilson, a researcher at Loma Linda University, in California. Wilson is studying how vagus nerve stimulation (VNS) could help pre-term babies who develop lung infections. "Nearly every one of our chronic diseases, including cancer, Alzheimer's, Parkinson's, chronic arthritis and rheumatoid arthritis, and depression and chronic pain…could benefit from an appropriate stimulator," he said.
It's unfortunate that Kelly got her device only after her large intestine was gone. SetPoint Medical, a privately held California company founded to develop electronic treatments for chronic autoimmune diseases, has announced early positive results with VNS for both Crohn's and rheumatoid arthritis.
As SetPoint's chief medical officer, David Chernoff, put it, "We're hacking into the nervous system to activate a system that is already there," an approach that, he said, could work "on many diseases that are pain- and inflammation-based." Inflammation plays a role in much modern illness, including depression and obesity. The FDA already has approved VNS for both, using surgically implanted devices similar to pacemakers. (GammaCore is external.)
The history of VNS implants goes back to 1997, when the FDA approved one for treating epilepsy and researchers noticed that it rapidly lifted depression in epileptic patients. By 2005, the agency had approved an implant for treatment-resistant depression. (Insurance companies declined to reimburse the approach and it didn't take off, but that might change: in February, the Center for Medicare and Medicaid Services asked for more data to evaluate coverage.) In 2015, the FDA approved an implant in the abdomen to regulate appetite signals and help obese people lose weight.
The link to inflammation had emerged a decade earlier, when researchers at the Feinstein Institute for Medical Research, in Manhasset, New York, demonstrated that stimulating the nerve with electricity in rats suppressed the production of cytokines, a signaling protein important in the immune system. The researchers developed a concept of a hard-wired pathway, through the vagus nerve, between the immune and nervous system. That pathway, they argued, regulates inflammation. While other researchers argue that VNS is helpful by other routes, there is clear evidence that, one way or another, it does affect immunity.
At the same time, investors are seeking alternatives to drugs.
The Feinstein rat research concluded that it took only a minute a day of stimulation and tiny amounts of energy to activate an anti-inflammatory reflex. This means you can use devices "the size of a coffee bean," said Chernoff, much less clunky than current pacemakers—and advances in electronic technology are making them possible.
At the same time, investors are seeking alternatives to drugs. "There's been a push back on drug pricing," noted Lisa Rhoads, a managing director at Easton Capital Investment Group, in New York, which supported electroCore, "and so many unintended consequences."
In 2016, the U.S. National Institutes of Health began pumping money into relevant research, in a program called "Stimulating Peripheral Activity to Relieve Conditions," which focuses on "understanding peripheral nerves — nerves that connect the brain and spinal cord to the rest of the body — and how their electrical signals control internal organ function."
GlaxoSmithKline formed Galvani Bioelectronics with Google to study miniature implants. It had already invested in Action Potential Venture Capital, in Cambridge, Massachusetts, which holds SetPoint and seven other companies "that are all targeting a nerve to treat a chronic disease," noted partner Imran Eba. "I see a future in which bioelectronics medicine is competing directly with drugs," he said.
Treating the body with electricity could bring more ease and lower costs. Many people with serious auto-immune disease, for example, have to inject themselves with drugs that cost $60,000 a year. SetPoint's implant would cost less and only need charging once a week, using a charger worn around the neck, Chernoff said. The company receives notices remotely and can monitor compliance.
Implants also allow the treatment to target a nerve precisely, which could be important with Parkinson's, chronic pain, and depression, observed James Cavuoto, editor and publisher of Neurotech Reports. They may also allow for more fine-turning. "In general, the industry is looking for signals, biomarkers that indicate when is the right time to turn on and turn off the stimulation. It could dramatically increase the effectiveness of the therapy and conserve battery life," he said.
Eventually, external devices could receive data from biomarkers as well. "It could be something you wear on your wrist," Cavuoto noted. Bluetooth-enabled devices could communicate with phones or laptops for data capture. External devices don't require surgery and put the patient in charge. "In the future you'll see more customer specification: Give the patient a tablet or phone app that lets them track and modify their parameters, within a range. With digital devices we have an enormous capability to customize therapies and collect data and get feedback that can be fed back to the clinician," Cavuoto said.
Slow deep breathing, the traditional mind-body intervention, is "like watching Little League. What we're doing is Major League."
It's even possible to stimulate the vagus through the ear, where one branch of the bundle of fibers begins. In a fetus, the tissue that becomes the ear is also part of the vagus nerve, and that one bit remains. "It's the same point as the acupuncture point," explained Mark George, a psychiatrist and pioneer researcher in depression at Medical University of South Carolina in Charleston. "Acupuncture figured out years ago by trial and error what we're just learning about now."
Slow deep breathing, the traditional mind-body intervention, also affects the vagus nerve in positive ways, but gently. "That's like watching Little League," Staats, the co-founder of electroCore, said. "What we're doing is Major League."
In ten years, researcher Wilson suggested, you could be wearing "a little ear cuff" that monitors your basic autonomic tone, a heart-attack risk measure governed in part by the vagus nerve. If your tone looked iffy, the stimulator would intervene, he said, "and improve your mood, cognition, and health."
In the meantime, we can take some long slow breaths, read Whitman, and sing.
“Siri, Read My Mind”: A New Device Lets Users Think Commands
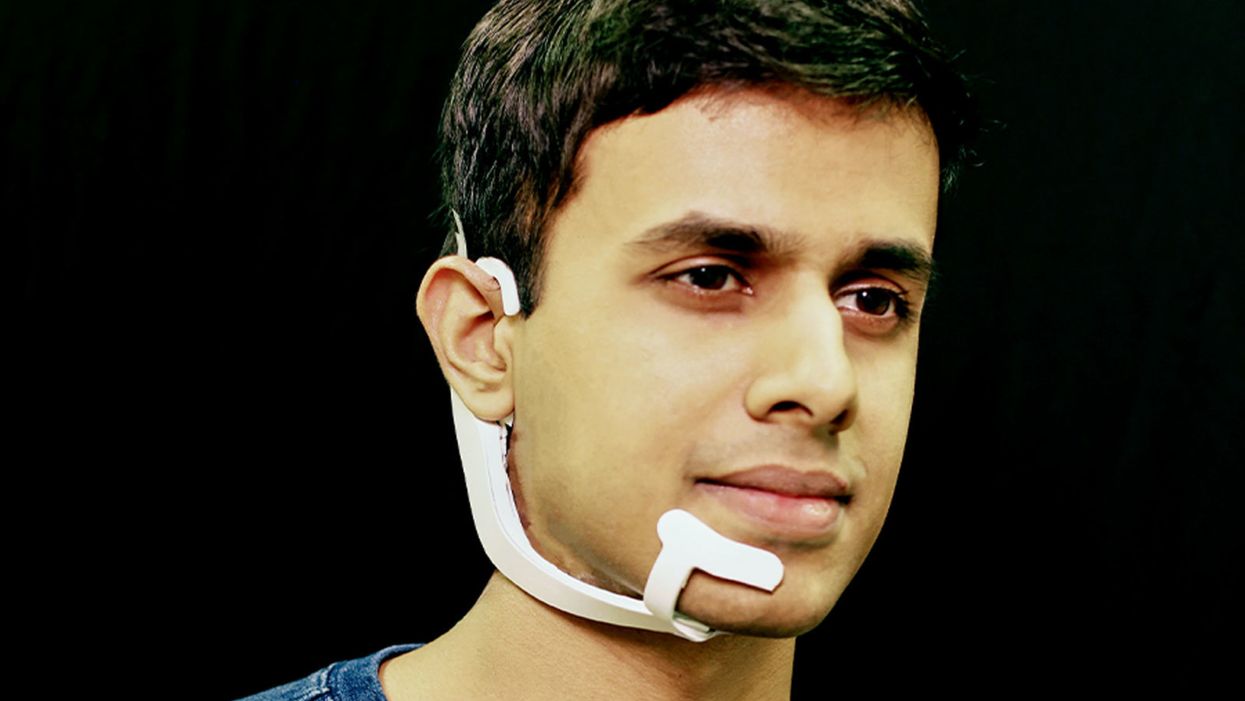
Arnav Kapur, a researcher in the Fluid Interfaces Group at MIT, wears an earlier prototype of the AlterEgo. A newer version is more discreet.
Sometime in the near future, we won't need to type on a smartphone or computer to silently communicate our thoughts to others.
"We're moving as fast as possible to get the technology right, to get the ethics right, to get everything right."
In fact, the devices themselves will quietly understand our intentions and express them to other people. We won't even need to move our mouths.
That "sometime in the near future" is now.
At the recent TED Conference, MIT student and TED Fellow Arnav Kapur was onstage with a colleague doing the first live public demo of his new technology. He was showing how you can communicate with a computer using signals from your brain. The usually cool, erudite audience seemed a little uncomfortable.
"If you look at the history of computing, we've always treated computers as external devices that compute and act on our behalf," Kapur said. "What I want to do is I want to weave computing, AI and Internet as part of us."
His colleague started up a device called AlterEgo. Thin like a sticker, AlterEgo picks up signals in the mouth cavity. It recognizes the intended speech and processes it through the built-in AI. The device then gives feedback to the user directly through bone conduction: It vibrates your inner ear drum and gives you a response meshing with your normal hearing.
Onstage, the assistant quietly thought of a question: "What is the weather in Vancouver?" Seconds later, AlterEgo told him in his ear. "It's 50 degrees and rainy here in Vancouver," the assistant announced.
AlterEgo essentially gives you a built-in Siri.
"We don't have a deadline [to go to market], but we're moving as fast as possible to get the technology right, to get the ethics right, to get everything right," Kapur told me after the talk. "We're developing it both as a general purpose computer interface and [in specific instances] like on the clinical side or even in people's homes."
Nearly-telepathic communication actually makes sense now. About ten years ago, the Apple iPhone replaced the ubiquitous cell phone keyboard with a blank touchscreen. A few years later, Google Glass put computer screens into a simple lens. More recently, Amazon Alexa and Microsoft Cortana have dropped the screen and gone straight for voice control. Now those voices are getting closer to our minds and may even become indistinguishable in the future.
"We knew the voice market was growing, like with getting map locations, and audio is the next frontier of user interfaces," says Dr. Rupal Patel, Founder and CEO of VocalID. The startup literally gives voices to the voiceless, particularly people unable to speak because of illness or other circumstances.
"We start with [our database of] human voices, then train our deep learning technology to learn the pattern of speech… We mix voices together from our voice bank, so it's not just Damon's voice, but three or five voices. They are different enough to blend it into a voice that does not exist today – kind of like a face morph."
The VocalID customer then has a voice as unique as he or she is, mixed together like a Sauvignon blend. It is a surrogate voice for those of us who cannot speak, just as much as AlterEgo is a surrogate companion for our brains.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon."
Voice equality will become increasingly important as Siri, Alexa and voice-based interfaces become the dominant communication method.
It may feel odd to view your voice as a privilege, but as the world becomes more voice-activated, there will be a wider gap between the speakers and the voiceless. Picture going shopping without access to the Internet or trying to eat healthily when your neighborhood is a food desert. And suffering from vocal difficulties is more common than you might think. In fact, according to government statistics, around 7.5 million people in the U.S. have trouble using their voices.
While voice communication appears to be here to stay, at least for now, a more radical shift to mind-controlled communication is not necessarily inevitable. Tech futurist Wagner James Au, for one, is dubious.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon. Generation Z has grown up with smartphones and games like Fortnite, so I don't see them quickly switching to a new form factor. It's still unclear if even head-mounted AR/VR displays will see mass adoption, and mind-reading devices are a far greater physical imposition on the user."
How adopters use the newest brain impulse-reading, voice-altering technology is a much more complicated discussion. This spring, a video showed U.S. House Speaker Nancy Pelosi stammering and slurring her words at a press conference. The problem is that it didn't really happen: the video was manufactured and heavily altered from the original source material.
So-called deepfake videos use computer algorithms to capture the visual and vocal cues of an individual, and then the creator can manipulate it to say whatever it wants. Deepfakes have already created false narratives in the political and media systems – and these are only videos. Newer tech is making the barrier between tech and our brains, if not our entire identity, even thinner.
"Last year," says Patel of VocalID, "we did penetration testing with our voices on banks that use voice control – and our generation 4 system is even tricky for you and me to identify the difference (between real and fake). As a forward-thinking company, we want to prevent risk early on by watermarking voices, creating a detector of false voices, and so on." She adds, "The line will become more blurred over time."
Onstage at TED, Kapur reassured the audience about who would be in the driver's seat. "This is why we designed the system to deliberately record from the peripheral nervous system, which is why the control in all situations resides with the user."
And, like many creators, he quickly shifted back to the possibilities. "What could the implications of something like this be? Imagine perfectly memorizing things, where you perfectly record information that you silently speak, and then hear them later when you want to, internally searching for information, crunching numbers at speeds computers do, silently texting other people."
"The potential," he concluded, "could be far-reaching."