Eight Big Medical and Science Trends to Watch in 2021

Promising developments underway include advancements in gene and cell therapy, better testing for COVID, and a renewed focus on climate change.
The world as we know it has forever changed. With a greater focus on science and technology than before, experts in the biotech and life sciences spaces are grappling with what comes next as SARS-CoV-2, the coronavirus that causes the COVID-19 illness, has spread and mutated across the world.
Even with vaccines being distributed, so much still remains unknown.
Jared Auclair, Technical Supervisor for the Northeastern University's Life Science Testing Center in Burlington, Massachusetts, guides a COVID testing lab that cranks out thousands of coronavirus test results per day. His lab is also focused on monitoring the quality of new cell and gene therapy products coming to the market.
Here are trends Auclair and other experts are watching in 2021.
Better Diagnostic Testing for COVID
Expect improvements in COVID diagnostic testing and the ability to test at home.
There are currently three types of coronavirus tests. The molecular test—also known as the RT-PCR test, detects the virus's genetic material, and is highly accurate, but it can take days to receive results. There are also antibody tests, done through a blood draw, designed to test whether you've had COVID in the past. Finally, there's the quick antigen test that isn't as accurate as the PCR test, but can identify if people are going to infect others.
Last month, Lucira Health secured the U.S. FDA Emergency Use Authorization for the first prescription molecular diagnostic test for COVID-19 that can be performed at home. On December 15th, the Ellume Covid-19 Home Test received authorization as the first over-the-counter COVID-19 diagnostic antigen test that can be done at home without a prescription. The test uses a nasal swab that is connected to a smartphone app and returns results in 15-20 minutes. Similarly, the BinaxNOW COVID-19 Ag Card Home Test received authorization on Dec. 16 for its 15-minute antigen test that can be used within the first seven days of onset of COIVD-19 symptoms.
Home testing has the possibility to impact the pandemic pretty drastically, Auclair says, but there are other considerations: the type and timing of test that is administered, how expensive is the test (and if it is financially feasible for the general public) and the ability of a home test taker to accurately administer the test.
"The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Ideally, everyone would frequently get tested, but that would mean the cost of a single home test—which is expected to be around $30 or more—would need to be much cheaper, more in the $5 range.
Auclair expects "innovations in the diagnostic space to explode" with the need for more accurate, inexpensive, quicker COVID tests. Auclair foresees innovations to be at first focused on COVID point-of-care testing, but he expects improvements within diagnostic testing for other types of viruses and diseases too.
"We still need more testing to get the pandemic under control, likely over the next 12 months," Auclair says. "The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Rise of mRNA-based Vaccines and Therapies
A year ago, vaccines weren't being talked about like they are today.
"But clearly vaccines are the talk of the town," Auclair says. "The reason we got a vaccine so fast was there was so much money thrown at it."
A vaccine can take more than 10 years to fully develop, according to the World Economic Forum. Prior to the new COVID vaccines, which were remarkably developed and tested in under a year, the fastest vaccine ever made was for mumps -- and it took four years.
"Normally you have to produce a protein. This is typically done in eggs. It takes forever," says Catherine Dulac, a neuroscientist and developmental biologist at Harvard University who won the 2021 Breakthrough Prize in Life Sciences. "But an mRNA vaccine just enabled [us] to skip all sorts of steps [compared with burdensome conventional manufacturing] and go directly to a product that can be injected into people."
Non-traditional medicines based on genetic research are in their infancy. With mRNA-based vaccines hitting the market for the first time, look for more vaccines to be developed for whatever viruses we don't currently have vaccines for, like dengue virus and Ebola, Auclair says.
"There's a whole bunch of things that could be explored now that haven't been thought about in the past," Auclair says. "It could really be a game changer."
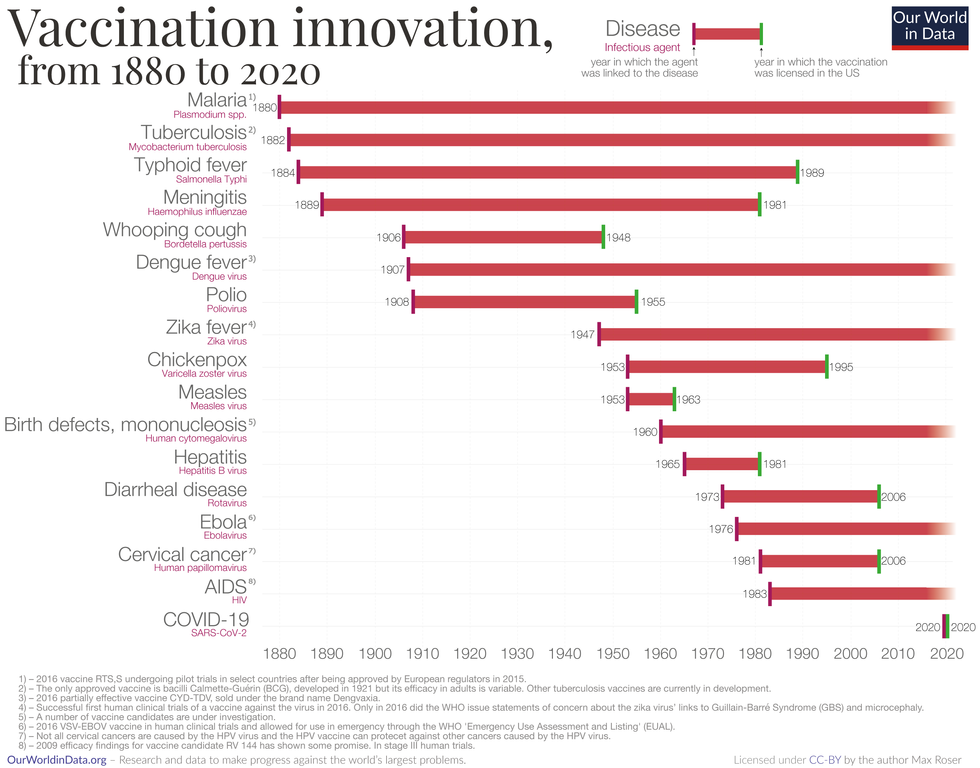
Vaccine Innovation over the last 140 years.
Max Roser/Our World in Data (Creative Commons license)
Advancements in Cell and Gene Therapies
CRISPR, a type of gene editing, is going to be huge in 2021, especially after the Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna in October for pioneering the technology.
Right now, CRISPR isn't completely precise and can cause deletions or rearrangements of DNA.
"It's definitely not there yet, but over the next year it's going to get a lot closer and you're going to have a lot of momentum in this space," Auclair says. "CRISPR is one of the technologies I'm most excited about and 2021 is the year for it."
Gene therapies are typically used on rare genetic diseases. They work by replacing the faulty dysfunctional genes with corrected DNA codes.
"Cell and gene therapies are really where the field is going," Auclair says. "There is so much opportunity....For the first time in our life, in our existence as a species, we may actually be able to cure disease by using [techniques] like gene editing, where you cut in and out of pieces of DNA that caused a disease and put in healthy DNA," Auclair says.
For example, Spinal Muscular Atrophy is a rare genetic disorder that leads to muscle weakness, paralysis and death in children by age two. As of last year, afflicted children can take a gene therapy drug called Zolgensma that targets the missing or nonworking SMN1 gene with a new copy.
Another recent breakthrough uses gene editing for sickle cell disease. Victoria Gray, a mom from Mississippi who was exclusively followed by NPR, was the first person in the United States to be successfully treated for the genetic disorder with the help of CRISPR. She has continued to improve since her landmark treatment on July 2, 2019 and her once-debilitating pain has greatly eased.
"This is really a life-changer for me," she told NPR. "It's magnificent."
"You are going to see bigger leaps in gene therapies."
Look out also for improvements in cell therapies, but on a much lesser scale.
Cell therapies remove immune cells from a person or use cells from a donor. The cells are modified or cultured in lab, multiplied by the millions and then injected back into patients. These include stem cell therapies as well as CAR-T cell therapies, which are typically therapies of last resort and used in cancers like leukemia, Auclair says.
"You are going to see bigger leaps in gene therapies," Auclair says. "It's being heavily researched and we understand more about how to do gene therapies. Cell therapies will lie behind it a bit because they are so much more difficult to work with right now."
More Monoclonal Antibody Therapies
Look for more customized drugs to personalize medicine even more in the biotechnology space.
In 2019, the FDA anticipated receiving more than 200 Investigational New Drug (IND) applications in 2020. But with COVID, the number of INDs skyrocketed to 6,954 applications for the 2020 fiscal year, which ended September 30, 2020, according to the FDA's online tracker. Look for antibody therapies to play a bigger role.
Monoclonal antibodies are lab-grown proteins that mimic or enhance the immune system's response to fight off pathogens, like viruses, and they've been used to treat cancer. Now they are being used to treat patients with COVID-19.
President Donald Trump received a monoclonal antibody cocktail, called REGEN-COV2, which later received FDA emergency use authorization.
A newer type of monoclonal antibody therapy is Antibody-Drug Conjugates, also called ADCs. It's something we're going to be hearing a lot about in 2021, Auclair says.
"Antibody-Drug Conjugates is a monoclonal antibody with a chemical, we consider it a chemical warhead on it," Auclair says. "The monoclonal antibody binds to a specific antigen in your body or protein and delivers a chemical to that location and kills the infected cell."
Moving Beyond Male-Centric Lab Testing
Scientific testing for biology has, until recently, focused on testing males. Dulac, a Howard Hughes Medical Investigator and professor of molecular and cellular biology at Harvard University, challenged that idea to find brain circuitry behind sex-specific behaviors.
"For the longest time, until now, all the model systems in biology, are male," Dulac says. "The idea is if you do testing on males, you don't need to do testing on females."
Clinical models are done in male animals, as well as fundamental research. Because biological research is always done on male models, Dulac says the outcomes and understanding in biology is geared towards understanding male biology.
"All the drugs currently on the market and diagnoses of diseases are biased towards the understanding of male biology," Dulac says. "The diagnostics of diseases is way weaker in women than men."
That means the treatment isn't necessarily as good for women as men, she says, including what is known and understood about pain medication.
"So pain medication doesn't work well in women," Dulac says. "It works way better in men. It's true for almost all diseases that I know. Why? because you have a science that is dominated by males."
Although some in the scientific community challenge that females are not interesting or too complicated with their hormonal variations, Dulac says that's simply not true.
"There's absolutely no reason to decide 50% of life forms are interesting and the other 50% are not interesting. What about looking at both?" says Dulac, who was awarded the $3 million Breakthrough Prize in Life Sciences in September for connecting specific neural mechanisms to male and female parenting behaviors.
Disease Research on Single Cells
To better understand how diseases manifest in the body's cell and tissues, many researchers are looking at single-cell biology. Cells are the most fundamental building blocks of life. Much still needs to be learned.
"A remarkable development this year is the massive use of analysis of gene expression and chromosomal regulation at the single-cell level," Dulac says.
Much is focused on the Human Cell Atlas (HCA), a global initiative to map all cells in healthy humans and to better identify which genes associated with diseases are active in a person's body. Most estimates put the number of cells around 30 trillion.
Dulac points to work being conducted by the Cell Census Network (BICCN) Brain Initiative, an initiative by the National Institutes of Health to come up with an atlas of cell types in mouse, human and non-human primate brains, and the Chan Zuckerberg Initiative's funding of single-cell biology projects, including those focused on single-cell analysis of inflammation.
"Our body and our brain are made of a large number of cell types," Dulac says. "The ability to explore and identify differences in gene expression and regulation in massively multiplex ways by analyzing millions of cells is extraordinarily important."
Converting Plastics into Food
Yep, you heard it right, plastics may eventually be turned into food. The Defense Advanced Research Projects Agency, better known as DARPA, is funding a project—formally titled "Production of Macronutrients from Thermally Oxo-Degraded Wastes"—and asking researchers how to do this.
"When I first heard about this challenge, I thought it was absolutely absurd," says Dr. Robert Brown, director of the Bioeconomy Institute at Iowa State University and the project's principal investigator, who is working with other research partners at the University of Delaware, Sandia National Laboratories, and the American Institute of Chemical Engineering (AIChE)/RAPID Institute.
But then Brown realized plastics will slowly start oxidizing—taking in oxygen—and microorganisms can then consume it. The oxidation process at room temperature is extremely slow, however, which makes plastics essentially not biodegradable, Brown says.
That changes when heat is applied at brick pizza oven-like temperatures around 900-degrees Fahrenheit. The high temperatures get compounds to oxidize rapidly. Plastics are synthetic polymers made from petroleum—large molecules formed by linking many molecules together in a chain. Heated, these polymers will melt and crack into smaller molecules, causing them to vaporize in a process called devolatilization. Air is then used to cause oxidation in plastics and produce oxygenated compounds—fatty acids and alcohols—that microorganisms will eat and grow into single-cell proteins that can be used as an ingredient or substitute in protein-rich foods.
"The caveat is the microorganisms must be food-safe, something that we can consume," Brown says. "Like supplemental or nutritional yeast, like we use to brew beer and to make bread or is used in Australia to make Vegemite."
What do the microorganisms look like? For any home beer brewers, it's the "gunky looking stuff you'd find at the bottom after the fermentation process," Brown says. "That's cellular biomass. Like corn grown in the field, yeast or other microorganisms like bacteria can be harvested as macro-nutrients."
Brown says DARPA's ReSource program has challenged all the project researchers to find ways for microorganisms to consume any plastics found in the waste stream coming out of a military expeditionary force, including all the packaging of food and supplies. Then the researchers aim to remake the plastic waste into products soldiers can use, including food. The project is in the first of three phases.
"We are talking about polyethylene, polypropylene, like PET plastics used in water bottles and converting that into macronutrients that are food," says Brown.
Renewed Focus on Climate Change
The Union of Concerned Scientists say carbon dioxide levels are higher today than any point in at least 800,000 years.
"Climate science is so important for all of humankind. It is critical because the quality of life of humans on the planet depends on it."
Look for technology to help locate large-scale emitters of carbon dioxide, including sensors on satellites and artificial intelligence to optimize energy usage, especially in data centers.
Other technologies focus on alleviating the root cause of climate change: emissions of heat-trapping gasses that mainly come from burning fossil fuels.
Direct air carbon capture, an emerging effort to capture carbon dioxide directly from ambient air, could play a role.
The technology is in the early stages of development and still highly uncertain, says Peter Frumhoff, director of science and policy at Union of Concerned Scientists. "There are a lot of questions about how to do that at sufficiently low costs...and how to scale it up so you can get carbon dioxide stored in the right way," he says, and it can be very energy intensive.
One of the oldest solutions is planting new forests, or restoring old ones, which can help convert carbon dioxide into oxygen through photosynthesis. Hence the Trillion Trees Initiative launched by the World Economic Forum. Trees are only part of the solution, because planting trees isn't enough on its own, Frumhoff says. That's especially true, since 2020 was the year that human-made, artificial stuff now outweighs all life on earth.
More research is also going into artificial photosynthesis for solar fuels. The U.S. Department of Energy awarded $100 million in 2020 to two entities that are conducting research. Look also for improvements in battery storage capacity to help electric vehicles, as well as back-up power sources for solar and wind power, Frumhoff says.
Another method to combat climate change is solar geoengineering, also called solar radiation management, which reflects sunlight back to space. The idea stems from a volcanic eruption in 1991 that released a tremendous amount of sulfate aerosol particles into the stratosphere, reflecting the sunlight away from Earth. The planet cooled by a half degree for nearly a year, Frumhoff says. However, he acknowledges, "there's a lot of things we don't know about the potential impacts and risks" involved in this controversial approach.
Whatever the approach, scientific solutions to climate change are attracting renewed attention. Under President Trump, the White House Office of Science and Technology Policy didn't have an acting director for almost two years. Expect that to change when President-elect Joe Biden takes office.
"Climate science is so important for all of humankind," Dulac says. "It is critical because the quality of life of humans on the planet depends on it."
Nobel Prize goes to technology for mRNA vaccines
Katalin Karikó, pictured, and Drew Weissman won the Nobel Prize for advances in mRNA research that led to the first Covid vaccines.
When Drew Weissman received a call from Katalin Karikó in the early morning hours this past Monday, he assumed his longtime research partner was calling to share a nascent, nagging idea. Weissman, a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania, and Karikó, a professor at Szeged University and an adjunct professor at UPenn, both struggle with sleep disturbances. Thus, middle-of-the-night discourses between the two, often over email, has been a staple of their friendship. But this time, Karikó had something more pressing and exciting to share: They had won the 2023 Nobel Prize in Physiology or Medicine.
The work for which they garnered the illustrious award and its accompanying $1,000,000 cash windfall was completed about two decades ago, wrought through long hours in the lab over many arduous years. But humanity collectively benefited from its life-saving outcome three years ago, when both Moderna and Pfizer/BioNTech’s mRNA vaccines against COVID were found to be safe and highly effective at preventing severe disease. Billions of doses have since been given out to protect humans from the upstart viral scourge.
“I thought of going somewhere else, or doing something else,” said Katalin Karikó. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
Unlocking the power of mRNA
Weissman and Karikó unlocked mRNA vaccines for the world back in the early 2000s when they made a key breakthrough. Messenger RNA molecules are essentially instructions for cells’ ribosomes to make specific proteins, so in the 1980s and 1990s, researchers started wondering if sneaking mRNA into the body could trigger cells to manufacture antibodies, enzymes, or growth agents for protecting against infection, treating disease, or repairing tissues. But there was a big problem: injecting this synthetic mRNA triggered a dangerous, inflammatory immune response resulting in the mRNA’s destruction.
While most other researchers chose not to tackle this perplexing problem to instead pursue more lucrative and publishable exploits, Karikó stuck with it. The choice sent her academic career into depressing doldrums. Nobody would fund her work, publications dried up, and after six years as an assistant professor at the University of Pennsylvania, Karikó got demoted. She was going backward.
“I thought of going somewhere else, or doing something else,” Karikó told Stat in 2020. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
A tale of tenacity
Collaborating with Drew Weissman, a new professor at the University of Pennsylvania, in the late 1990s helped provide Karikó with the tenacity to continue. Weissman nurtured a goal of developing a vaccine against HIV-1, and saw mRNA as a potential way to do it.
“For the 20 years that we’ve worked together before anybody knew what RNA is, or cared, it was the two of us literally side by side at a bench working together,” Weissman said in an interview with Adam Smith of the Nobel Foundation.
In 2005, the duo made their 2023 Nobel Prize-winning breakthrough, detailing it in a relatively small journal, Immunity. (Their paper was rejected by larger journals, including Science and Nature.) They figured out that chemically modifying the nucleoside bases that make up mRNA allowed the molecule to slip past the body’s immune defenses. Karikó and Weissman followed up that finding by creating mRNA that’s more efficiently translated within cells, greatly boosting protein production. In 2020, scientists at Moderna and BioNTech (where Karikó worked from 2013 to 2022) rushed to craft vaccines against COVID, putting their methods to life-saving use.
The future of vaccines
Buoyed by the resounding success of mRNA vaccines, scientists are now hurriedly researching ways to use mRNA medicine against other infectious diseases, cancer, and genetic disorders. The now ubiquitous efforts stand in stark contrast to Karikó and Weissman’s previously unheralded struggles years ago as they doggedly worked to realize a shared dream that so many others shied away from. Katalin Karikó and Drew Weissman were brave enough to walk a scientific path that very well could have ended in a dead end, and for that, they absolutely deserve their 2023 Nobel Prize.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Scientists turn pee into power in Uganda
With conventional fuel cells as their model, researchers learned to use similar chemical reactions to make a fuel from microbes in pee.
At the edge of a dirt road flanked by trees and green mountains outside the town of Kisoro, Uganda, sits the concrete building that houses Sesame Girls School, where girls aged 11 to 19 can live, learn and, at least for a while, safely use a toilet. In many developing regions, toileting at night is especially dangerous for children. Without electrical power for lighting, kids may fall into the deep pits of the latrines through broken or unsteady floorboards. Girls are sometimes assaulted by men who hide in the dark.
For the Sesame School girls, though, bright LED lights, connected to tiny gadgets, chased the fears away. They got to use new, clean toilets lit by the power of their own pee. Some girls even used the light provided by the latrines to study.
Urine, whether animal or human, is more than waste. It’s a cheap and abundant resource. Each day across the globe, 8.1 billion humans make 4 billion gallons of pee. Cows, pigs, deer, elephants and other animals add more. By spending money to get rid of it, we waste a renewable resource that can serve more than one purpose. Microorganisms that feed on nutrients in urine can be used in a microbial fuel cell that generates electricity – or "pee power," as the Sesame girls called it.
Plus, urine contains water, phosphorus, potassium and nitrogen, the key ingredients plants need to grow and survive. Human urine could replace about 25 percent of current nitrogen and phosphorous fertilizers worldwide and could save water for gardens and crops. The average U.S. resident flushes a toilet bowl containing only pee and paper about six to seven times a day, which adds up to about 3,500 gallons of water down per year. Plus cows in the U.S. produce 231 gallons of the stuff each year.
Pee power
A conventional fuel cell uses chemical reactions to produce energy, as electrons move from one electrode to another to power a lightbulb or phone. Ioannis Ieropoulos, a professor and chair of Environmental Engineering at the University of Southampton in England, realized the same type of reaction could be used to make a fuel from microbes in pee.
Bacterial species like Shewanella oneidensis and Pseudomonas aeruginosa can consume carbon and other nutrients in urine and pop out electrons as a result of their digestion. In a microbial fuel cell, one electrode is covered in microbes, immersed in urine and kept away from oxygen. Another electrode is in contact with oxygen. When the microbes feed on nutrients, they produce the electrons that flow through the circuit from one electrod to another to combine with oxygen on the other side. As long as the microbes have fresh pee to chomp on, electrons keep flowing. And after the microbes are done with the pee, it can be used as fertilizer.
These microbes are easily found in wastewater treatment plants, ponds, lakes, rivers or soil. Keeping them alive is the easy part, says Ieropoulos. Once the cells start producing stable power, his group sequences the microbes and keeps using them.
Like many promising technologies, scaling these devices for mass consumption won’t be easy, says Kevin Orner, a civil engineering professor at West Virginia University. But it’s moving in the right direction. Ieropoulos’s device has shrunk from the size of about three packs of cards to a large glue stick. It looks and works much like a AAA battery and produce about the same power. By itself, the device can barely power a light bulb, but when stacked together, they can do much more—just like photovoltaic cells in solar panels. His lab has produced 1760 fuel cells stacked together, and with manufacturing support, there’s no theoretical ceiling, he says.
Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofit into urban wastewater utilities.
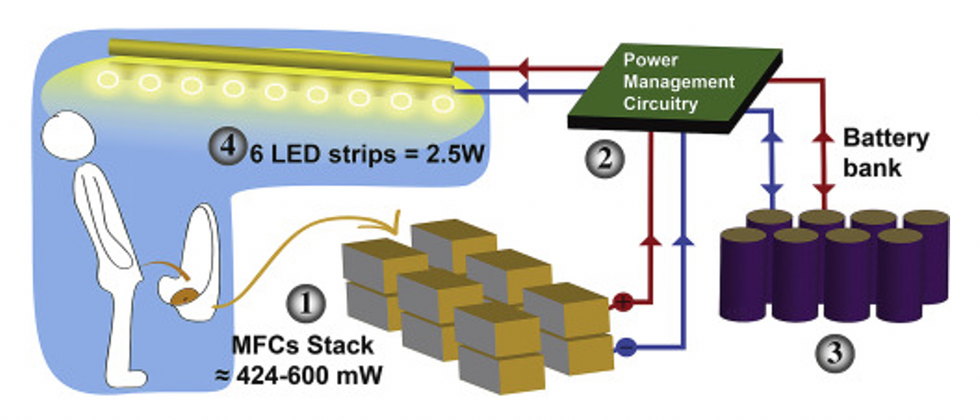
This image shows how the pee-powered system works. Pee feeds bacteria in the stack of fuel cells (1), which give off electrons (2) stored in parallel cylindrical cells (3). These cells are connected to a voltage regulator (4), which smooths out the electrical signal to ensure consistent power to the LED strips lighting the toilet.
Courtesy Ioannis Ieropoulos
Key to the long-term success of any urine reclamation effort, says Orner, is avoiding what he calls “parachute engineering”—when well-meaning scientists solve a problem with novel tech and then abandon it. “The way around that is to have either the need come from the community or to have an organization in a community that is committed to seeing a project operate and maintained,” he says.
Success with urine reclamation also depends on the economy. “If energy prices are low, it may not make sense to recover energy,” says Orner. “But right now, fertilizer prices worldwide are generally pretty high, so it may make sense to recover fertilizer and nutrients.” There are obstacles, too, such as few incentives for builders to incorporate urine recycling into new construction. And any hiccups like leaks or waste seepage will cost builders money and reputation. Right now, Orner says, the risks are just too high.
Despite the challenges, Ieropoulos envisions a future in which urine is passed through microbial fuel cells at wastewater treatment plants, retrofitted septic tanks, and building basements, and is then delivered to businesses to use as agricultural fertilizers. Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofitted into urban wastewater utilities where they can make electricity from the effluent. And unlike solar cells, which are a common target of theft in some areas, nobody wants to steal a bunch of pee.
When Ieropoulos’s team returned to wrap up their pilot project 18 months later, the school’s director begged them to leave the fuel cells in place—because they made a major difference in students’ lives. “We replaced it with a substantial photovoltaic panel,” says Ieropoulos, They couldn’t leave the units forever, he explained, because of intellectual property reasons—their funders worried about theft of both the technology and the idea. But the photovoltaic replacement could be stolen, too, leaving the girls in the dark.
The story repeated itself at another school, in Nairobi, Kenya, as well as in an informal settlement in Durban, South Africa. Each time, Ieropoulos vowed to return. Though the pandemic has delayed his promise, he is resolute about continuing his work—it is a moral and legal obligation. “We've made a commitment to ourselves and to the pupils,” he says. “That's why we need to go back.”
Urine as fertilizer
Modern day industrial systems perpetuate the broken cycle of nutrients. When plants grow, they use up nutrients the soil. We eat the plans and excrete some of the nutrients we pass them into rivers and oceans. As a result, farmers must keep fertilizing the fields while our waste keeps fertilizing the waterways, where the algae, overfertilized with nitrogen, phosphorous and other nutrients grows out of control, sucking up oxygen that other marine species need to live. Few global communities remain untouched by the related challenges this broken chain create: insufficient clean water, food, and energy, and too much human and animal waste.
The Rich Earth Institute in Vermont runs a community-wide urine nutrient recovery program, which collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms.
One solution to this broken cycle is reclaiming urine and returning it back to the land. The Rich Earth Institute in Vermont is one of several organizations around the world working to divert and save urine for agricultural use. “The urine produced by an adult in one day contains enough fertilizer to grow all the wheat in one loaf of bread,” states their website.
Notably, while urine is not entirely sterile, it tends to harbor fewer pathogens than feces. That’s largely because urine has less organic matter and therefore less food for pathogens to feed on, but also because the urinary tract and the bladder have built-in antimicrobial defenses that kill many germs. In fact, the Rich Earth Institute says it’s safe to put your own urine onto crops grown for home consumption. Nonetheless, you’ll want to dilute it first because pee usually has too much nitrogen and can cause “fertilizer burn” if applied straight without dilution. Other projects to turn urine into fertilizer are in progress in Niger, South Africa, Kenya, Ethiopia, Sweden, Switzerland, The Netherlands, Australia, and France.
Eleven years ago, the Institute started a program that collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms. By 2021, the program included 180 donors producing over 12,000 gallons of urine each year. This urine is helping to fertilize hay fields at four partnering farms. Orner, the West Virginia professor, sees it as a success story. “They've shown how you can do this right--implementing it at a community level scale."

