Eight Big Medical and Science Trends to Watch in 2021

Promising developments underway include advancements in gene and cell therapy, better testing for COVID, and a renewed focus on climate change.
The world as we know it has forever changed. With a greater focus on science and technology than before, experts in the biotech and life sciences spaces are grappling with what comes next as SARS-CoV-2, the coronavirus that causes the COVID-19 illness, has spread and mutated across the world.
Even with vaccines being distributed, so much still remains unknown.
Jared Auclair, Technical Supervisor for the Northeastern University's Life Science Testing Center in Burlington, Massachusetts, guides a COVID testing lab that cranks out thousands of coronavirus test results per day. His lab is also focused on monitoring the quality of new cell and gene therapy products coming to the market.
Here are trends Auclair and other experts are watching in 2021.
Better Diagnostic Testing for COVID
Expect improvements in COVID diagnostic testing and the ability to test at home.
There are currently three types of coronavirus tests. The molecular test—also known as the RT-PCR test, detects the virus's genetic material, and is highly accurate, but it can take days to receive results. There are also antibody tests, done through a blood draw, designed to test whether you've had COVID in the past. Finally, there's the quick antigen test that isn't as accurate as the PCR test, but can identify if people are going to infect others.
Last month, Lucira Health secured the U.S. FDA Emergency Use Authorization for the first prescription molecular diagnostic test for COVID-19 that can be performed at home. On December 15th, the Ellume Covid-19 Home Test received authorization as the first over-the-counter COVID-19 diagnostic antigen test that can be done at home without a prescription. The test uses a nasal swab that is connected to a smartphone app and returns results in 15-20 minutes. Similarly, the BinaxNOW COVID-19 Ag Card Home Test received authorization on Dec. 16 for its 15-minute antigen test that can be used within the first seven days of onset of COIVD-19 symptoms.
Home testing has the possibility to impact the pandemic pretty drastically, Auclair says, but there are other considerations: the type and timing of test that is administered, how expensive is the test (and if it is financially feasible for the general public) and the ability of a home test taker to accurately administer the test.
"The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Ideally, everyone would frequently get tested, but that would mean the cost of a single home test—which is expected to be around $30 or more—would need to be much cheaper, more in the $5 range.
Auclair expects "innovations in the diagnostic space to explode" with the need for more accurate, inexpensive, quicker COVID tests. Auclair foresees innovations to be at first focused on COVID point-of-care testing, but he expects improvements within diagnostic testing for other types of viruses and diseases too.
"We still need more testing to get the pandemic under control, likely over the next 12 months," Auclair says. "The vaccine roll-out will not eliminate the need for testing until late 2021 or early 2022."
Rise of mRNA-based Vaccines and Therapies
A year ago, vaccines weren't being talked about like they are today.
"But clearly vaccines are the talk of the town," Auclair says. "The reason we got a vaccine so fast was there was so much money thrown at it."
A vaccine can take more than 10 years to fully develop, according to the World Economic Forum. Prior to the new COVID vaccines, which were remarkably developed and tested in under a year, the fastest vaccine ever made was for mumps -- and it took four years.
"Normally you have to produce a protein. This is typically done in eggs. It takes forever," says Catherine Dulac, a neuroscientist and developmental biologist at Harvard University who won the 2021 Breakthrough Prize in Life Sciences. "But an mRNA vaccine just enabled [us] to skip all sorts of steps [compared with burdensome conventional manufacturing] and go directly to a product that can be injected into people."
Non-traditional medicines based on genetic research are in their infancy. With mRNA-based vaccines hitting the market for the first time, look for more vaccines to be developed for whatever viruses we don't currently have vaccines for, like dengue virus and Ebola, Auclair says.
"There's a whole bunch of things that could be explored now that haven't been thought about in the past," Auclair says. "It could really be a game changer."
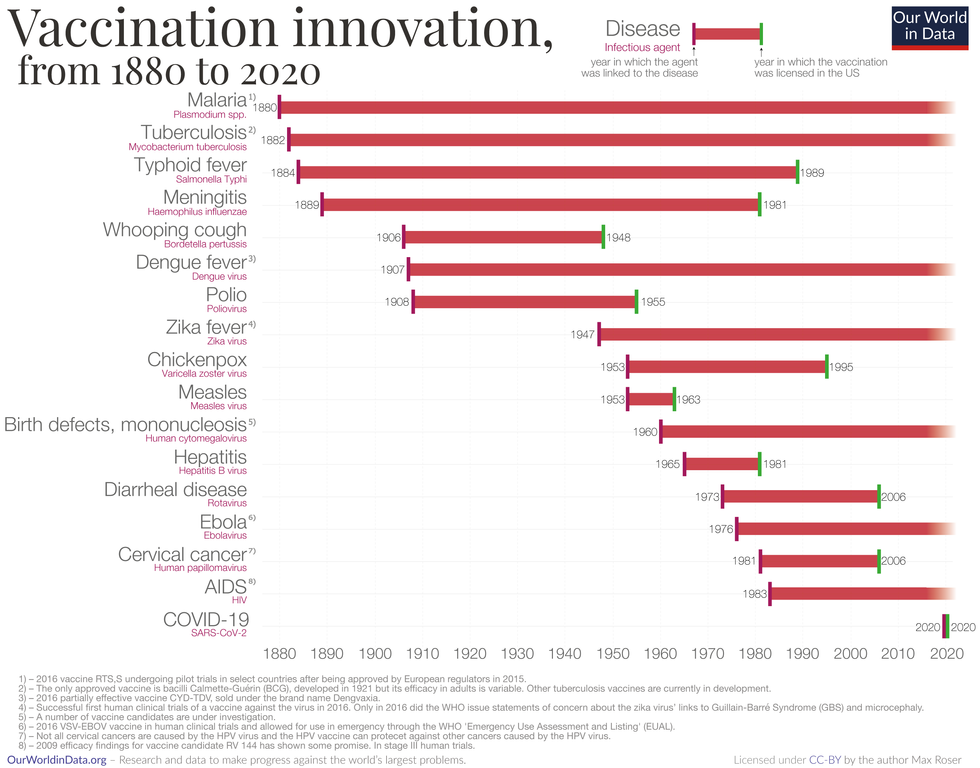
Vaccine Innovation over the last 140 years.
Max Roser/Our World in Data (Creative Commons license)
Advancements in Cell and Gene Therapies
CRISPR, a type of gene editing, is going to be huge in 2021, especially after the Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna in October for pioneering the technology.
Right now, CRISPR isn't completely precise and can cause deletions or rearrangements of DNA.
"It's definitely not there yet, but over the next year it's going to get a lot closer and you're going to have a lot of momentum in this space," Auclair says. "CRISPR is one of the technologies I'm most excited about and 2021 is the year for it."
Gene therapies are typically used on rare genetic diseases. They work by replacing the faulty dysfunctional genes with corrected DNA codes.
"Cell and gene therapies are really where the field is going," Auclair says. "There is so much opportunity....For the first time in our life, in our existence as a species, we may actually be able to cure disease by using [techniques] like gene editing, where you cut in and out of pieces of DNA that caused a disease and put in healthy DNA," Auclair says.
For example, Spinal Muscular Atrophy is a rare genetic disorder that leads to muscle weakness, paralysis and death in children by age two. As of last year, afflicted children can take a gene therapy drug called Zolgensma that targets the missing or nonworking SMN1 gene with a new copy.
Another recent breakthrough uses gene editing for sickle cell disease. Victoria Gray, a mom from Mississippi who was exclusively followed by NPR, was the first person in the United States to be successfully treated for the genetic disorder with the help of CRISPR. She has continued to improve since her landmark treatment on July 2, 2019 and her once-debilitating pain has greatly eased.
"This is really a life-changer for me," she told NPR. "It's magnificent."
"You are going to see bigger leaps in gene therapies."
Look out also for improvements in cell therapies, but on a much lesser scale.
Cell therapies remove immune cells from a person or use cells from a donor. The cells are modified or cultured in lab, multiplied by the millions and then injected back into patients. These include stem cell therapies as well as CAR-T cell therapies, which are typically therapies of last resort and used in cancers like leukemia, Auclair says.
"You are going to see bigger leaps in gene therapies," Auclair says. "It's being heavily researched and we understand more about how to do gene therapies. Cell therapies will lie behind it a bit because they are so much more difficult to work with right now."
More Monoclonal Antibody Therapies
Look for more customized drugs to personalize medicine even more in the biotechnology space.
In 2019, the FDA anticipated receiving more than 200 Investigational New Drug (IND) applications in 2020. But with COVID, the number of INDs skyrocketed to 6,954 applications for the 2020 fiscal year, which ended September 30, 2020, according to the FDA's online tracker. Look for antibody therapies to play a bigger role.
Monoclonal antibodies are lab-grown proteins that mimic or enhance the immune system's response to fight off pathogens, like viruses, and they've been used to treat cancer. Now they are being used to treat patients with COVID-19.
President Donald Trump received a monoclonal antibody cocktail, called REGEN-COV2, which later received FDA emergency use authorization.
A newer type of monoclonal antibody therapy is Antibody-Drug Conjugates, also called ADCs. It's something we're going to be hearing a lot about in 2021, Auclair says.
"Antibody-Drug Conjugates is a monoclonal antibody with a chemical, we consider it a chemical warhead on it," Auclair says. "The monoclonal antibody binds to a specific antigen in your body or protein and delivers a chemical to that location and kills the infected cell."
Moving Beyond Male-Centric Lab Testing
Scientific testing for biology has, until recently, focused on testing males. Dulac, a Howard Hughes Medical Investigator and professor of molecular and cellular biology at Harvard University, challenged that idea to find brain circuitry behind sex-specific behaviors.
"For the longest time, until now, all the model systems in biology, are male," Dulac says. "The idea is if you do testing on males, you don't need to do testing on females."
Clinical models are done in male animals, as well as fundamental research. Because biological research is always done on male models, Dulac says the outcomes and understanding in biology is geared towards understanding male biology.
"All the drugs currently on the market and diagnoses of diseases are biased towards the understanding of male biology," Dulac says. "The diagnostics of diseases is way weaker in women than men."
That means the treatment isn't necessarily as good for women as men, she says, including what is known and understood about pain medication.
"So pain medication doesn't work well in women," Dulac says. "It works way better in men. It's true for almost all diseases that I know. Why? because you have a science that is dominated by males."
Although some in the scientific community challenge that females are not interesting or too complicated with their hormonal variations, Dulac says that's simply not true.
"There's absolutely no reason to decide 50% of life forms are interesting and the other 50% are not interesting. What about looking at both?" says Dulac, who was awarded the $3 million Breakthrough Prize in Life Sciences in September for connecting specific neural mechanisms to male and female parenting behaviors.
Disease Research on Single Cells
To better understand how diseases manifest in the body's cell and tissues, many researchers are looking at single-cell biology. Cells are the most fundamental building blocks of life. Much still needs to be learned.
"A remarkable development this year is the massive use of analysis of gene expression and chromosomal regulation at the single-cell level," Dulac says.
Much is focused on the Human Cell Atlas (HCA), a global initiative to map all cells in healthy humans and to better identify which genes associated with diseases are active in a person's body. Most estimates put the number of cells around 30 trillion.
Dulac points to work being conducted by the Cell Census Network (BICCN) Brain Initiative, an initiative by the National Institutes of Health to come up with an atlas of cell types in mouse, human and non-human primate brains, and the Chan Zuckerberg Initiative's funding of single-cell biology projects, including those focused on single-cell analysis of inflammation.
"Our body and our brain are made of a large number of cell types," Dulac says. "The ability to explore and identify differences in gene expression and regulation in massively multiplex ways by analyzing millions of cells is extraordinarily important."
Converting Plastics into Food
Yep, you heard it right, plastics may eventually be turned into food. The Defense Advanced Research Projects Agency, better known as DARPA, is funding a project—formally titled "Production of Macronutrients from Thermally Oxo-Degraded Wastes"—and asking researchers how to do this.
"When I first heard about this challenge, I thought it was absolutely absurd," says Dr. Robert Brown, director of the Bioeconomy Institute at Iowa State University and the project's principal investigator, who is working with other research partners at the University of Delaware, Sandia National Laboratories, and the American Institute of Chemical Engineering (AIChE)/RAPID Institute.
But then Brown realized plastics will slowly start oxidizing—taking in oxygen—and microorganisms can then consume it. The oxidation process at room temperature is extremely slow, however, which makes plastics essentially not biodegradable, Brown says.
That changes when heat is applied at brick pizza oven-like temperatures around 900-degrees Fahrenheit. The high temperatures get compounds to oxidize rapidly. Plastics are synthetic polymers made from petroleum—large molecules formed by linking many molecules together in a chain. Heated, these polymers will melt and crack into smaller molecules, causing them to vaporize in a process called devolatilization. Air is then used to cause oxidation in plastics and produce oxygenated compounds—fatty acids and alcohols—that microorganisms will eat and grow into single-cell proteins that can be used as an ingredient or substitute in protein-rich foods.
"The caveat is the microorganisms must be food-safe, something that we can consume," Brown says. "Like supplemental or nutritional yeast, like we use to brew beer and to make bread or is used in Australia to make Vegemite."
What do the microorganisms look like? For any home beer brewers, it's the "gunky looking stuff you'd find at the bottom after the fermentation process," Brown says. "That's cellular biomass. Like corn grown in the field, yeast or other microorganisms like bacteria can be harvested as macro-nutrients."
Brown says DARPA's ReSource program has challenged all the project researchers to find ways for microorganisms to consume any plastics found in the waste stream coming out of a military expeditionary force, including all the packaging of food and supplies. Then the researchers aim to remake the plastic waste into products soldiers can use, including food. The project is in the first of three phases.
"We are talking about polyethylene, polypropylene, like PET plastics used in water bottles and converting that into macronutrients that are food," says Brown.
Renewed Focus on Climate Change
The Union of Concerned Scientists say carbon dioxide levels are higher today than any point in at least 800,000 years.
"Climate science is so important for all of humankind. It is critical because the quality of life of humans on the planet depends on it."
Look for technology to help locate large-scale emitters of carbon dioxide, including sensors on satellites and artificial intelligence to optimize energy usage, especially in data centers.
Other technologies focus on alleviating the root cause of climate change: emissions of heat-trapping gasses that mainly come from burning fossil fuels.
Direct air carbon capture, an emerging effort to capture carbon dioxide directly from ambient air, could play a role.
The technology is in the early stages of development and still highly uncertain, says Peter Frumhoff, director of science and policy at Union of Concerned Scientists. "There are a lot of questions about how to do that at sufficiently low costs...and how to scale it up so you can get carbon dioxide stored in the right way," he says, and it can be very energy intensive.
One of the oldest solutions is planting new forests, or restoring old ones, which can help convert carbon dioxide into oxygen through photosynthesis. Hence the Trillion Trees Initiative launched by the World Economic Forum. Trees are only part of the solution, because planting trees isn't enough on its own, Frumhoff says. That's especially true, since 2020 was the year that human-made, artificial stuff now outweighs all life on earth.
More research is also going into artificial photosynthesis for solar fuels. The U.S. Department of Energy awarded $100 million in 2020 to two entities that are conducting research. Look also for improvements in battery storage capacity to help electric vehicles, as well as back-up power sources for solar and wind power, Frumhoff says.
Another method to combat climate change is solar geoengineering, also called solar radiation management, which reflects sunlight back to space. The idea stems from a volcanic eruption in 1991 that released a tremendous amount of sulfate aerosol particles into the stratosphere, reflecting the sunlight away from Earth. The planet cooled by a half degree for nearly a year, Frumhoff says. However, he acknowledges, "there's a lot of things we don't know about the potential impacts and risks" involved in this controversial approach.
Whatever the approach, scientific solutions to climate change are attracting renewed attention. Under President Trump, the White House Office of Science and Technology Policy didn't have an acting director for almost two years. Expect that to change when President-elect Joe Biden takes office.
"Climate science is so important for all of humankind," Dulac says. "It is critical because the quality of life of humans on the planet depends on it."
New tech for prison reform spreads to 11 states
The U.S. has the highest incarceration rate in the world, costing $182 billion per year, partly because its antiquated data systems often fail to identify people who should be released. A tech nonprofit is trying to change that.
A new non-profit called Recidiviz is using data technology to reduce the size of the U.S. criminal justice system. The bi-coastal company (SF and NYC) is currently working with 11 states to improve their systems and, so far, has helped remove nearly 69,000 people — ones left floundering in jail or on parole when they should have been released.
“The root cause is fragmentation,” says Clementine Jacoby, 31, a software engineer who worked at Google before co-founding Recidiviz in 2019. In the 1970s and 80s, the U.S. built a series of disconnected data systems, and this patchwork is still being used by criminal justice authorities today. It requires parole officers to manually calculate release dates, leading to errors in many cases. “[They] have done everything they need to do to earn their release, but they're still stuck in the system,” Jacoby says.
Recidiviz has built a platform that connects the different databases, with the goal of identifying people who are already qualified for release but remain behind bars or on supervision. “Think of Recidiviz like Google Maps,” says Jacoby, who worked on Maps when she was at the tech giant. Google Maps takes in data from different sources – satellite images, street maps, local business data — and organizes it into one easy view. “Recidiviz does something similar with criminal justice data,” Jacoby explains, “making it easy to identify people eligible to come home or to move to less intensive levels of supervision.”
People like Jacoby’s uncle. His experience with incarceration is what inspired her passion for criminal justice reform in the first place.
The problems are vast
The U.S. has the highest incarceration rate in the world — 2 million people according to the watchdog group, Prison Policy Initiative — at a cost of $182 billion a year. The numbers could be a lot lower if not for an array of problems including inaccurate sentencing calculations, flawed algorithms and parole violations laws.
Sentencing miscalculations
To determine eligibility for release, the current system requires corrections officers to check 21 different requirements spread across five different databases for each of the 90 to 100 people under their supervision. These manual calculations are time prohibitive, says Jacoby, and fall victim to human error.
In addition, Recidiviz found that policies aimed at helping to reduce the prison population don’t always work correctly. A key example is time off for good behavior laws that allow inmates to earn one day off for every 30 days of good behavior. Some states' data systems are built to calculate time off as one day per month of good behavior, rather than per day. Over the course of a decade-long sentence, Jacoby says these miscalculations can lead to a huge discrepancy in the calculated release data and the actual release date.
Algorithms
Commercial algorithm-based software systems for risk assessment continue to be widely used in the criminal justice system, even though a 2018 study published in Science Advances exposed their limitations. After the study went viral, it took three years for the Justice Department to issue a report on their own flawed algorithms used to reduce the federal prison population as part of the 2018 First Step Act. The program, it was determined, overestimated the risk of putting inmates of color into early-release programs.
Despite its name, Recidiviz does not build these types of algorithms for predicting recidivism, or whether someone will commit another crime after being released from prison. Rather, Jacoby says the company’s "descriptive analytics” approach is specifically intended to weed out incarceration inequalities and avoid algorithmic pitfalls.
Parole violation laws
Research shows that 350,000 people a year — about a quarter of the total prison population — are sent back not because they’ve committed another crime, but because they’ve broken a specific rule of their probation. “Things that wouldn't send you or I to prison, but would send someone on parole,” such as crossing county lines or being in the presence of alcohol when they shouldn’t be, are inflating the prison population, says Jacoby.
It’s personal for the co-founder and CEO
“I grew up with an uncle who went into the prison system,” Jacoby says. At 19, he was sentenced to ten years in prison for a non-violent crime. A few months after being released from jail, he was sent back for a non-violent parole violation.
“For my family, the fact that one in four prison admissions are driven not by a crime but by someone who's broken a rule on probation and parole was really profound because that happened to my uncle,” Jacoby says. The experience led her to begin studying criminal justice in high school, then college. She continued her dive into how the criminal justice system works as part of her Passion Project while at Google, a program that allows employees to spend 20 percent of their time on pro-bono work. Two colleagues whose family members had also been stuck in the system joined her.
As part of the project, Jacoby interviewed hundreds of people involved in the criminal justice system. “Those on the right, those on the left, agreed that bad data was slowing down reform,” she says. Their research brought them to North Dakota where they began to understand the root of the problem. The corrections department is making “huge, consequential decisions every day [without] … the data,” Jacoby says. In a new video by Recidiviz not yet released, Jacoby recounts her exchange with the state’s director of corrections who told her, “‘It’s not that we have the data and we just don’t know how to make it public; we don’t have the information you think we have.'"
A mock-up (with fake data) of the types of dashboards and insights that Recidiviz provides to state governments.
Recidiviz
As a software engineer, Jacoby says the comment made no sense to her — until she witnessed it first-hand. “We spent a lot of time driving around in cars with corrections directors and parole officers watching them use these incredibly taxing, frankly terrible, old data systems,” Jacoby says.
As they weeded through thousands of files — some computerized, some on paper — they unearthed the consequences of bad data: Hundreds of people in prison well past their release date and thousands more whose release from parole was delayed because of minor paperwork issues. They found individuals stuck in parole because they hadn’t checked one last item off their eligibility list — like simply failing to provide their parole officer with a paystub. And, even when parolees advocated for themselves, the archaic system made it difficult for their parole officers to confirm their eligibility, so they remained in the system. Jacoby and her team also unpacked specific policies that drive racial disparities — such as fines and fees.
The Solution
It’s more than a trivial technical challenge to bring the incomplete, fragmented data onto a 21st century data platform. It takes months for Recidiviz to sift through a state’s information systems to connect databases “with the goal of tracking a person all the way through their journey and find out what’s working for 18- to 25-year-old men, what’s working for new mothers,” explains Jacoby in the video.
TED Talk: How bad data traps people in the U.S. justice system

TED Fellow Clementine Jacoby's TED Talk went live on Jan. 13. It describes how we can fix bad data in the criminal justice system, "bringing thousands of people home, reducing costs and improving public safety along the way."
Clementine Jacoby • TED2022
Ojmarrh Mitchell, an associate professor in the School of Criminology and Criminal Justice at Arizona State University, who is not involved with the company, says what Recidiviz is doing is “remarkable.” His perspective goes beyond academic analysis. In his pre-academic years, Mitchell was a probation officer, working within the framework of the “well known, but invisible” information sharing issues that plague criminal justice departments. The flexibility of Recidiviz’s approach is what makes it especially innovative, he says. “They identify the specific gaps in each jurisdiction and tailor a solution for that jurisdiction.”
On the downside, the process used by Recidiviz is “a bit opaque,” Mitchell says, with few details available on how Recidiviz designs its tools and tracks outcomes. By sharing more information about how its actions lead to progress in a given jurisdiction, Recidiviz could help reformers in other places figure out which programs have the best potential to work well.
The eleven states in which Recidiviz is working include California, Colorado, Maine, Michigan, Missouri, Pennsylvania and Tennessee. And a pilot program launched last year in Idaho, if scaled nationally, with could reduce the number of people in the criminal justice system by a quarter of a million people, Jacoby says. As part of the pilot, rather than relying on manual calculations, Recidiviz is equipping leaders and the probation officers with actionable information with a few clicks of an app that Recidiviz built.
Mitchell is disappointed that there’s even the need for Recidiviz. “This is a problem that government agencies have a responsibility to address,” he says. “But they haven’t.” For one company to come along and fill such a large gap is “remarkable.”
How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”

