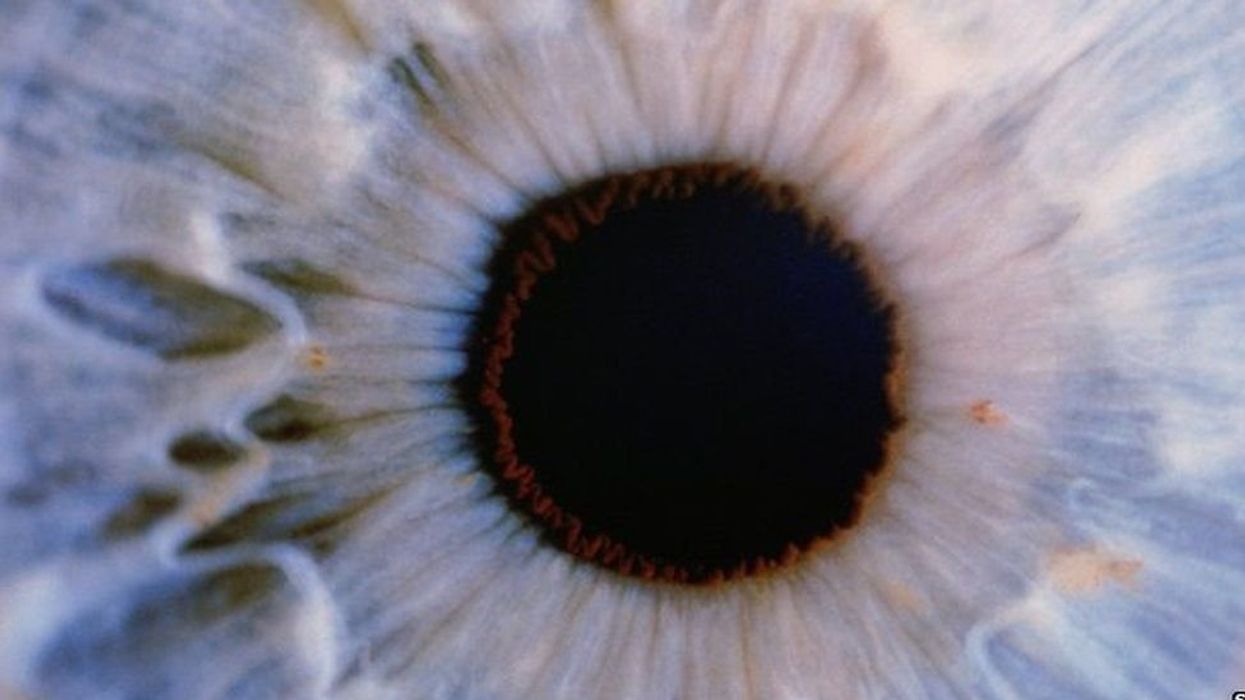
Gene therapy helps restore teen’s vision for first time
Doctors used new eye drops to treat a rare genetic disorder.
Story by Freethink
For the first time, a topical gene therapy — designed to heal the wounds of people with “butterfly skin disease” — has been used to restore a person’s vision, suggesting a new way to treat genetic disorders of the eye.
The challenge: Up to 125,000 people worldwide are living with dystrophic epidermolysis bullosa (DEB), an incurable genetic disorder that prevents the body from making collagen 7, a protein that helps strengthen the skin and other connective tissues.Without collagen 7, the skin is incredibly fragile — the slightest friction can lead to the formation of blisters and scarring, most often in the hands and feet, but in severe cases, also the eyes, mouth, and throat.
This has earned DEB the nickname of “butterfly skin disease,” as people with it are said to have skin as delicate as a butterfly’s wings.
The gene therapy: In May 2023, the FDA approved Vyjuvek, the first gene therapy to treat DEB.
Vyjuvek uses an inactivated herpes simplex virus to deliver working copies of the gene for collagen 7 to the body’s cells. In small trials, 65 percent of DEB-caused wounds sprinkled with it healed completely, compared to just 26 percent of wounds treated with a placebo.
“It was like looking through thick fog.” -- Antonio Vento Carvajal.
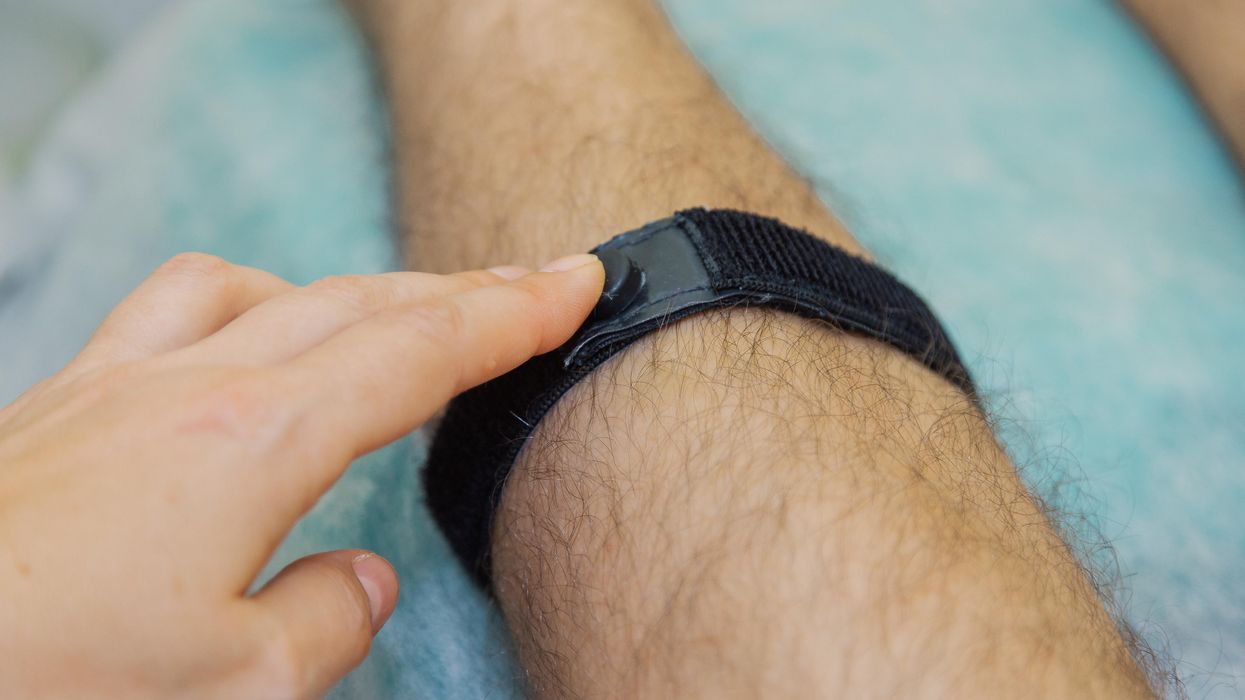
The patient: Antonio Vento Carvajal, a 14 year old living in Florida, was one of the trial participants to benefit from Vyjuvek, which was developed by Pittsburgh-based pharmaceutical company Krystal Biotech.
While the topical gene therapy could help his skin, though, it couldn’t do anything to address the severe vision loss Antonio experienced due to his DEB. He’d undergone multiple surgeries to have scar tissue removed from his eyes, but due to his condition, the blisters keep coming back.
“It was like looking through thick fog,” said Antonio, noting how his impaired vision made it hard for him to play his favorite video games. “I had to stand up from my chair, walk over, and get closer to the screen to be able to see.”
The idea: Encouraged by how Antonio’s skin wounds were responding to the gene therapy, Alfonso Sabater, his doctor at the Bascom Palmer Eye Institute, reached out to Krystal Biotech to see if they thought an alternative formula could potentially help treat his patient’s eyes.
The company was eager to help, according to Sabater, and after about two years of safety and efficacy testing, he had permission, under the FDA’s compassionate use protocol, to treat Antonio’s eyes with a version of the topical gene therapy delivered as eye drops.
The results: In August 2022, Sabater once again removed scar tissue from Antonio’s right eye, but this time, he followed up the surgery by immediately applying eye drops containing the gene therapy.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy. Don’t be afraid.” -- Yunielkys “Yuni” Carvajal.
The vision in Antonio’s eye steadily improved. By about eight months after the treatment, it was just slightly below average (20/25) and stayed that way. In March 2023, Sabater performed the same procedure on his young patient’s other eye, and the vision in it has also steadily improved.
“I’ve seen the transformation in Antonio’s life,” said Sabater. “He’s always been a happy kid. Now he’s very happy. He can function pretty much normally. He can read, he can study, he can play video games.”
Looking ahead: The topical gene therapy isn’t a permanent fix — it doesn’t alter Antonio’s own genes, so he has to have the eye drops reapplied every month. Still, that’s far less invasive than having to undergo repeated surgeries.
Sabater is now working with Krystal Biotech to launch trials of the eye drops in other patients, and not just those with DEB. By changing the gene delivered by the therapy, he believes it could be used to treat other eye disorders that are far more common — Fuchs’ dystrophy, for example, affects the vision of an estimated 300 million people over the age of 30.
Antonio’s mother, Yunielkys “Yuni” Carvajal, meanwhile, has said that having her son be the first to receive the eye drops was “very scary,” but she’s hopeful others will take a chance on new gene therapies if given the opportunity.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy,” she said. “Don’t be afraid.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.

In this week's Friday Five, research on a "smart" bandage for wounds, a breakthrough in fighting inflammation, the pros and cons of a new drug for Alzheimer's, benefits of the Mediterranean diet with a twist, and we've learned to recycle a plastic that was un-recyclable.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- Research on a "smart" bandage for wounds
- A breakthrough in fighting inflammation
- The pros and cons of a new drug for Alzheimer's
- Benefits of the Mediterranean diet - with a twist
- How to recycle a plastic that was un-recyclable
Sexually Transmitted Infections are on the rise. This drug could stop them.
Cases of gonorrhea, chlamydia and syphilis soared last year, but researchers are finding that a drug known as doxy seems to reduce the number of infections.
Sexually transmitted infections (STIs) are surging across the U.S. to 2.5 million cases in 2021 according to preliminary data from the CDC. A new prevention and treatment strategy now in clinical trials may provide a way to get a handle on them.
It's easy to overlook the soaring rates of gonorrhea, chlamydia, and syphilis because most of those infections have few or no symptoms and can be identified only through testing. But left untreated, they can lead to serious damage to nerves and tissue, resulting in infertility, blindness, and dementia. Infants developing in utero are particularly vulnerable.
Covid-19 played havoc with regular medical treatment and preventive care for many health problems, including STIs. After formal lockdowns ended, many people gradually became more socially engaged, with increases in sexual activity, and may have prioritized these activities over getting back in touch with their doctors.
A second blow to controlling STIs is that family planning clinics are closing left and right because of the Dobbs decision and legislation in many states that curtailed access to an abortion. Discussion has focused on abortion, but those same clinics also play a vital role in the diagnosis and treatment of STIs.
Routine public health is the neglected stepchild of medicine. It is called upon in times of crisis but as that crisis resolves, funding dries up. Labs have atrophied and personnel have been redirected to Covid, “so access to routine screening for STIs has been decimated,” says Jennifer Mahn, director of sexual and clinical health with the National Coalition of STD Directors.
A preview of what we likely are facing comes from Iowa. In 2017, the state legislature restricted funding to family health clinics in four counties, which closed their doors. A year later the statewide rate of gonorrhea skyrocketed from 83 to 153.7 cases per 100,000 people. “Iowa counties with clinic closures had a significantly larger increase,” according to a study published in JAMA. That scenario likely is playing out in countless other regions where access to sexual health care is shrinking; it will be many months before we have the data to know for sure.
A decades-old antibiotic finds a new purpose
Using drugs to protect against HIV, either as post exposure prophylaxis (PEP) or pre-exposure prophylaxis (PrEP), has proven to be quite successful. Researchers wondered if the same approach might be applied to other STIs. They focused on doxycycline, or doxy for short. One of the most commonly prescribed antibiotics in the U.S., it’s a member of the tetracycline family that has been on the market since 1967. It is so safe that it’s used to treat acne.
Two small studies using doxy suggested that it could work to prevent STIs. A handful of clinical trials by different researchers and funding sources set out to generate the additional evidence needed to prove their hypothesis and change the standard of care.
Senior researcher Victor Omollo, with the Kenya Medical Research Institute, noted, “These are prevention interventions that women can control on their own without having to seek or get consent from another person,” as is the case with condom use.
The first with results is the DoxyPEP study, conducted at two sexual health clinics in San Francisco and Seattle. It drew from a mix of transgender women and men who have sex with men, who had at least one diagnosed STI over the last year. The researchers divided the participants into two groups: one with people who were already HIV-positive and engaged in care, while the other group consisted of people who were on PrEP to prevent infection with HIV. For the active part of the study, a subset of the participants received doxy, and the rest of the participants did not.
The researchers intentionally chose to do the study in a population at the highest risk of having STIs, who were very health oriented, and “who were getting screened every three months or so as part of their PrEP program or their HIV care program,” says Connie Celum, a senior researcher at the University of Washington on the study.
Each member of the active group was given a supply of doxy and asked to take two pills within 72 hours of having sex where a condom was not used. The study was supposed to run for two years but, in May, it stopped halfway through, when a safety monitoring board looked at the data and recommended that it would be unethical to continue depriving the control group of the drug’s benefits.
Celum presented these preliminary results from the DoxyPEP study in July at the International AIDS Conference in Montreal. “We saw about a 56 percent reduction in gonorrhea, about 80 percent reduction in chlamydia and syphilis, so very significant reductions, and this is on a per quarter basis,” she told a later webinar.
In Kenya, another study is following a group of cisgender women who are taking the same two-pill regimen to prevent HIV, and the data from this research should become available in 2023. Senior researcher Victor Omollo, with the Kenya Medical Research Institute, noted that “these are prevention interventions that women can control on their own without having to seek or get consent from another person,” as is the case with condom use, another effective prevention tool.
Antibiotic resistance
Antibiotic resistance is a potentially big concern. About 25 percent of gonorrhea strains circulating in the U.S. are resistant to the tetracycline class of drugs, including doxy; rates are higher elsewhere. But resistance often is a matter of degree and can be overcome with a larger or longer dose of the drug, or perhaps with a switch to another drug or a two-drug combination.
Research has shown that an established bacterial infection is more difficult to treat because it is part of a biofilm, which can leave only a small portion or perhaps none of the cell surface exposed to a drug. But a new infection, even one where the bacteria is resistant to a drug, might still be vulnerable to that drug if it's used before the bacterial biofilm can be established. Preliminary data suggests that may be the case with doxyPEP and drug resistant gonorrhea; some but not all new drug resistant infections might be thwarted if they’re treated early enough.
“There are some tradeoffs” to these interventions, Celum says, and people may disagree on the cost of increased resistance balanced against the benefits of treating the STIs and reducing their spread within the community.
Resistance does not seem to be an issue yet for chlamydia and syphilis even though doxy has been a recommended treatment for decades, but a remaining question is whether broader use of doxy will directly worsen antibiotic resistance in gonorrhea, or promote it in other STIs. And how will it affect the gut microbiome?
In addition, Celum notes that we need to understand whether doxy will generate mutations in other bacteria that might contribute to drug resistance for gonorrhea, chlamydia or syphilis. The studies underway aim to provide data to answer these questions.
“There are some tradeoffs” to these interventions, Celum says, and people may disagree on the cost of increased resistance balanced against the benefits of treating the STIs and reducing their spread within the community. That might affect doctors' willingness to prescribe the drug.
Turning research into action
The CDC makes policy recommendations for prevention services such as taking doxy, requiring some and leaving others optional. Celum says the CDC will be reviewing information from her trial at a meeting in December, but probably will wait until that study is published before making recommendations, likely in 2023. The San Francisco Department of Public Health issued its own guidance on October 20th and anecdotally, some doctors around the country are beginning to issue prescriptions for doxy to select patients.
About half of new STIs occur in young people ages 15 to 24, a group that is least likely to regularly see a doctor. And sexual health remains a great taboo for many people who don't want such information on their health record for prying parents, employers or neighbors to find out.
“People will go out of their way and travel extensive distances just to avoid that,” says Mahn, the National Coalition director. “People identify locations where they feel safe, where they feel welcome, where they don't feel judged,” Mahn explains, such as community and family planning clinics. They understand those issues and have fees that vary depending on a person’s ability to pay.
Given that these clinics already are understaffed and underfunded, they will be hard pressed to expand services covering the labor intensive testing and monitoring of a doxyPEP regimen. Sexual health clinics don't even have a separate line item in the federal budget for health. That is something the National Association of STI Directors is pushing for in D.C.
DoxyPEP isn't a panacea, and it isn't for everyone. “We really want to try to reach that population who is most likely going to have an STI in the next year,” says Celum, “Because that's where you are going to have the biggest impact.”