Researchers Are Discovering How to Predict – and Maybe Treat — Pregnancy Complications Early On.
Katie Love cradles her newborn daughter, born after a bout with preeclampsia.
Katie Love wishes there was some way she could have been prepared. But there was no way to know, early in 2020, that her pregnancy would lead to terrifyingly high blood pressure and multiple hospital visits, ending in induced labor and a 56-hour-long, “nightmare” delivery at 37 weeks. Love, a social media strategist in Pittsburgh, had preeclampsia, a poorly understood and potentially deadly pregnancy complication that affects 1 in 25 pregnant women in the United States. But there was no blood test, no easy diagnostic marker to warn Love that this might happen. Even on her first visit to the emergency room, with sky-high blood pressure, doctors could not be certain preeclampsia was the cause.
In fact, the primary but imperfect indicators for preeclampsia — high blood pressure and protein in the urine — haven’t changed in decades. The Preeclampsia Foundation calls a simple, rapid test to predict or diagnose the condition “a key component needed in the fight.”
Another common pregnancy complication is preterm birth, which affects 1 in 10 U.S. pregnancies, but there are few options to predict that might happen, either.
“The best tool that obstetricians have at the moment is still a tape measure and a blood pressure cuff to diagnose whatever’s happening in your pregnancy,” says Fiona Kaper, a vice president at the DNA-sequencing company Illumina in San Diego.
The hunt for such specific biomarkers is now taking off, at Illumina and elsewhere, as scientists probe maternal blood for signs that could herald pregnancy problems. These same molecules offer clues that might lead to more specific treatments. So far, it’s clear that many complications start with the placenta, the temporary organ that transfers nutrients, oxygen and waste between mother and fetus, and that these problems often start well before symptoms arise. Researchers are using the latest stem-cell technology to better understand the causes of complications and test treatments.
Pressing Need
Obstetricians aren’t flying completely blind; medical history can point to high or low risk for pregnancy complications. But ultimately, “everybody who’s pregnant is at risk for preeclampsia,” says Sarosh Rana, chief of maternal-fetal medicine at University of Chicago Medicine and an advisor to the Preeclampsia Foundation. And the symptoms of the condition include problems like headache and swollen feet that overlap with those of pregnancy in general, complicating diagnoses.
The “holy grail" would be early, first-trimester biomarkers. If obstetricians and expecting parents could know, in the first few months of pregnancy, that preeclampsia is a risk, a pregnant woman could monitor her blood pressure at home and take-low dose aspirin that might stave it off.
There are a couple more direct tests physicians can turn to, but these are imperfect. For preterm labor, fetal fibronectin makes up a sort of glue that keeps the amniotic sac, which cushions the unborn baby, attached to the uterus. If it’s not present near a woman’s cervix, that’s a good indicator that she’s not in labor, and can be safely sent home, says Lauren Demosthenes, an obstetrician and senior medical director of the digital health company Babyscripts in Washington, D.C. But if fibronectin appears, it might or might not indicate preterm labor.
“What we want is a test that gives us a positive predictive [signal],” says Demosthenes. “I want to know, if I get it, is it really going to predict preterm birth, or is it just going to make us worry more and order more tests?” In fact, the fetal fibronectin test hasn’t been shown to improve pregnancy outcomes, and Demosthenes says it’s fallen out of favor in many clinics.
Similarly, there’s a blood test, based on the ratio of the amounts of two different proteins, that can rule out preeclampsia but not confirm it’s happening. It’s approved in many countries, though not the U.S.; studies are still ongoing. A positive test, which means “maybe preeclampsia,” still leaves doctors and parents-to-be facing excruciating decisions: If the mother’s life is in danger, delivering the baby can save her, but even a few more days in the uterus can promote the baby’s health. In Ireland, where the test is available, it’s not getting much use, says Patricia Maguire, director of the University College Dublin Institute for Discovery.
Maguire has identified proteins released by platelets that indicate pregnancy — the “most expensive pregnancy test in the world,” she jokes. She is now testing those markers in women with suspected preeclampsia.
The “holy grail,” says Maguire, would be early, first-trimester biomarkers. If obstetricians and expecting parents could know, in the first few months of pregnancy, that preeclampsia is a risk, a pregnant woman could monitor her blood pressure at home and take-low dose aspirin that might stave it off. Similarly, if a quick blood test indicated that preterm labor could happen, doctors could take further steps such as measuring the cervix and prescribing progesterone if it’s on the short side.
Biomarkers in Blood
It was fatherhood that drew Stephen Quake, a biophysicist at Stanford University in California, to the study of pregnancy biomarkers. His wife, pregnant with their first child in 2001, had a test called amniocentesis. That involves extracting a sample from within the uterus, using a 3–8-inch-long needle, for genetic testing. The test can identify genetic differences, such as Down syndrome, but also carries risks including miscarriage or infection. In this case, mom and baby were fine (Quake’s daughter is now a college student), but he found the diagnostic danger unacceptable.
Seeking a less invasive test, Quake in 2008 reported that there’s enough fetal DNA in the maternal bloodstream to diagnose Down syndrome and other genetic conditions. “Use of amniocentesis has plunged,” he says.
Then, recalling that his daughter was born three and a half weeks before her due date — and that Quake’s own mom claims he was a month late, which makes him think the due date must have been off — he started researching markers that could accurately assess a fetus’ age and predict the timing of labor. In this case, Quake was interested in RNA, not DNA, because it’s a signal of which genes the fetus’, placenta’s, and mother’s tissues are using to create proteins. Specifically, these are RNAs that have exited the cells that made them. Tissues can use such free RNAs as messages, wrapping them in membranous envelopes to travel the bloodstream to other body parts. Dying cells also release fragments containing RNAs. “A lot of information is in there,” says Kaper.
In a small study of 31 healthy pregnant women, published in 2018, Quake and collaborators discovered nine RNAs that could predict gestational age, which indicates due date, just as well as ultrasound. With another set of 38 women, including 13 who delivered early, the researchers discovered seven RNAs that predicted preterm labor up to two months in advance.
Quake notes that an RNA-based blood test is cheaper and more portable than ultrasound, so it might be useful in the developing world. A company he cofounded, Mirvie, Inc., is now analyzing RNA’s predictive value further, in thousands of diverse women. CEO and cofounder Maneesh Jain says that since preterm labor is so poorly understood, they’re sequencing RNAs that represent about 20,000 genes — essentially all the genes humans have — to find the very best biomarkers. “We don’t know enough about this field to guess what it might be,” he says. “We feel we’ve got to cast the net wide.”
Quake, and Mirvie, are now working on biomarkers for preeclampsia. In a recent preprint study, not yet reviewed by other experts, Quake’s Stanford team reported 18 RNAs that, measured before 16 weeks, correctly predicted preeclampsia 56–100% of the time.
Other researchers are taking a similar tack. Kaper’s team at Illumina was able to classify preeclampsia from bloodstream RNAs with 85 to 89% accuracy, though they didn’t attempt to predict it. And Louise Laurent, a maternal-fetal medicine specialist and researcher at the University of California, San Diego (UCSD), has defined several pairs of microRNAs — pint-sized RNAs that regulate other ones — in second-trimester blood samples that predict preeclampsia later on.
Placentas in a Dish
The RNAs that show up in these studies often come from genes used by the placenta. But they’re only signals that something’s wrong, not necessarily the root cause. “There still is not much known about what really causes major complications of pregnancy,” says Laurent.
The challenge is that placental problems likely occur early on, as the organ forms in the first trimester. For example, if the placenta did a poor job of building blood vessels through the uterine lining, it might cause preeclampsia later as the growing fetus tries to access more and more blood through insufficient vessels, leading to high blood pressure in the mother. “Everyone has kind of suspected that that is probably what goes wrong,” says Mana Parast, a pathologist and researcher at UCSD.
To see how a placenta first faltered, “you want to go back in time,” says Parast. It’s only recently become possible to do something akin to that: She and Laurent take cells from the umbilical cord (which is a genetic match for the placenta) at the end of pregnancy, and turn them into stem cells, which can become any kind of cell. They then nudge those stem cells to make new placenta cells in lab dishes. But when the researchers start with cells from an umbilical cord after preeclampsia, they find the stem cells struggle to even form proper placenta cells, or they develop abnormally. So yes, something seems to go wrong right at the beginning. Now, the team plans to use these cell cultures to study the microRNAs that indicate preeclampsia risk, and to look for medications that might reverse the problems, Parast says.
Biomarkers could lead to treatments. For example, one of the proteins that commercial preeclampsia diagnostic kits test for is called soluble Flt-1. It’s a sort of anti-growth factor, explains Rana, that can cause problems with blood vessels and thus high blood pressure. Getting rid of the extra Flt-1, then, might alleviate symptoms and keep the mother safe, giving the baby more time to develop. Indeed, a small trial that filtered this protein from the blood did lower blood pressure, allowing participants to keep their babies inside for a couple of weeks longer, researchers reported in 2011.
For pregnant women like Love, even advance warning would have been beneficial. Laurent and others envision a first-trimester blood test that would use different kinds of biomolecules — RNAs, proteins, whatever works best — to indicate whether a pregnancy is at low, medium, or high risk for common complications.
“I prefer to be prepared,” says Love, now the mother of a healthy little girl. “I just wouldn’t have been so thrown off by the whole thing.”
Science Has Given Us the Power to Undermine Nature's Deadliest Creature: Should We Use It?
The Aedes aegypti mosquito, which can carry devastating diseases, was recently engineered by a biotech company to have a genetic "kill switch" intended to crash the local population in the Florida Keys.
Lurking among the swaying palm trees, sugary sands and azure waters of the Florida Keys is the most dangerous animal on earth: the mosquito.
While there are thousands of varieties of mosquitoes, only a small percentage of them are responsible for causing disease. One of the leading culprits is Aedes aegypti, which thrives in the warm standing waters of South Florida, Central America and other tropical climes, and carries the viruses that cause yellow fever, dengue, chikungunya and Zika.
Dengue, a leading cause of death in many Asian and Latin American countries, causes bleeding and pain so severe that it's referred to as "breakbone fever." Chikungunya and yellow fever can both be fatal, and Zika, when contracted by a pregnant woman, can infect her fetus and cause devastating birth defects, including a condition called microcephaly. Babies born with this condition have abnormally small heads and lack proper brain development, which leads to profound, lifelong disabilities.
Decades of efforts to eradicate the disease-carrying Aedes aegypti mosquito from the Keys and other tropical locales have had limited impact. Since the advent of pesticides, homes and neighborhoods have been drenched with them, but after each spraying, the mosquito population quickly bounces back, and the pesticides have to be sprayed over and over. But thanks to genetic engineering, new approaches are underway that could possibly prove safer, cheaper and more effective than any pesticide.
One of those approaches involves, ironically, releasing more mosquitoes in the Florida Keys.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce.
British biotech company Oxitec has engineered male mosquitoes to have a genetic "kill-switch" that could potentially crash the local population of Aedes aegypti, at least in the short-term. The modified males that are being released are intended to mate with wild females.
Males don't bite; it's the female that's deadly, always seeking out blood to gorge on to help mature her eggs. After settling her filament-thin legs on her prey, she sinks a needlelike proboscis into the skin and sucks the blood until her translucent belly is bloated and glowing red.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce. In some experiments using genetically modified mosquitoes, the small number of females that survived were rendered unable to bite. The modification prevented the proboscis, the sickle-like needle that pierces the skin, from forming properly. But this isn't the case with Oxitec's mosquitoes; in the Oxitec release, the females simply die off before they can mate.
The modified mosquitoes are the second genetically engineered insect to be released in the U.S. by Oxitec. The first was a modified diamondback moth, an agricultural pest that doesn't bite humans. But with the mosquitoes, there are many questions about the long-term effects on wild ecosystems, other species in the food chain, and human health. With the Keys initiative, there has been vociferous opposition from environmental groups and some local residents, but some scientists and public health experts say that genetically modified insects pose less of a risk than the diseases they carry and the powerful, indiscriminant pesticides used to combat them.
Oxitec spent a decade developing the technology and engaging in a massive public education campaign before beginning the field test in April. Eventually, the company will release 750,000 of the insects from six locations on three islands of the Florida Keys. Although the release has been approved by the Environmental Protection Agency, the Florida Department of Agriculture and Consumer Services, and the Florida Keys Mosquito Control District, the company was never able to obtain unanimous approval among local residents, some of whom worry that the experiment could cause irreversible damage to the ecosystem.
The company has already begun distributing multiple blue and white boxes containing the eggs of thousands of the mosquitoes which, when water is added, will hatch legions of modified males.
There are a number of techniques available to genetically engineer animals and plants to minimize disease and maximize crop yields. According to Kevin Gorman, chief development officer for Oxitec, the company's mosquitoes were altered by injecting genetic material into the eggs, testing them, then re-injecting them if not enough of the new genes were incorporated into the developing embryos. "We insert genes, but take nothing away," he says.
Gorman points out that the Oxitec mosquitoes will only pass the kill-switch genes on to some of their offspring, and that they will die out fairly quickly. They should temporarily lessen diseases by reducing the local population of Aedes aegypti, but to have a long-term effect, repeated introductions of the altered mosquitoes would have to take place.
Critics say the Oxitec experiment is a precursor to a far more consequential, and more troubling development: the introduction of gene drives in modified species that aggressively tilt inheritance factors in a decided direction.
Gene Drives
Gene drives coupled with the recent development of the gene-editing technique, CRISPR-Cas9, promise to be far more targeted and powerful than previous gene altering efforts. Gene drives override the normal laws of inheritance by harnessing natural processes involved in reproduction. The technique targets small sections of the animal's DNA and replaces it with an altered allele, or trait-determining snippet. Normally, when two members of a species mate, the offspring have a 50 percent chance of receiving an allele because they will receive one from each parent. But in a gene drive, each offspring ends up getting two copies of a desired allele from a single parent—the modified parent. The method "drives" the modified DNA into up to 100 percent of the animals' offspring.
In the case of gene drive mosquitoes, the modified males will mate with wild females. Upon fertilization of the egg, the offspring will start off with one copy of the targeted allele from each parent. But an enzyme, called Cas9, is introduced and acts as a kind of molecular scissors to cut, or damage, the "wild" allele. Then the developing embryo's genetic repair mechanisms kick in and, to repair the damage, copy the undamaged allele from the modified parent. In this way, the offspring ends up with two copies of the modified allele, and it will pass the modification on to virtually all of its progeny.
There is some debate among researchers and others about what constitutes a gene drive, but leaders in the nascent field, such as Andrea Crisanti, generally agree that the defining factor is the heritability of a change introduced into a species. A gene drive is not a particular gene or suite of genes, but a program that proliferates in a species because it is inherited by virtually all offspring.
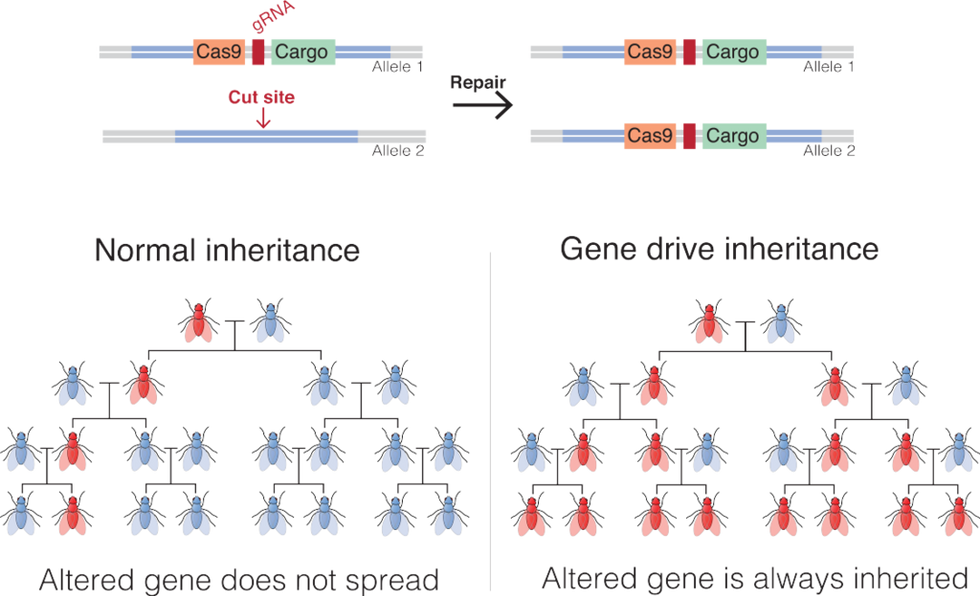
An illustration of how gene drives spread an altered gene through a population.
Mariuswalter, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Of the experts who spoke with Leaps.org for this article, there was disagreement on whether the Oxitec mosquitoes carry a gene drive, but Gorman says they don't because they carry no inheritance advantage. The mosquitoes have baked-in limitations on their potential impact on the tropical ecosystem because the kill-switch should only temporarily affect the local population of Aedes aegypti. The modified mosquitoes will die pretty quickly. But modified organisms that do carry gene drives have the potential to spread widely and persist for an unknown period of time.
Since it has such a reproductive advantage, animals modified by CRISPR and carrying gene drives can quickly replace wild species that compete with them. On the other hand, if the gene drive carries a kill-switch, it can theoretically cause a whole species to collapse.
This makes many people uneasy in an age of mass extinctions, when animals and ecosystems are already under extreme stress due to climate change and the ceaseless destruction of their habitats. Ecosystems are intricate, delicately balanced mosaics where one animal's competitor is another animal's food. The interconnectedness of nature is only partially understood and still contains many mysteries as to what effects human intervention could eventually cause.
But there's a compelling case to be made for the use of gene drives in general. Economies throughout the world are often based on the ecosystem and its animals, which rely on a natural food chain that was evolved over billions of years. But diseases carried by mosquitoes and other animals cause massive damage, both economically and in terms of human suffering.
Malaria alone is a case in point. In 2019, the World Health Organization reported 229 million cases of malaria, which led to 449,000 deaths worldwide. Over 70 percent of those deaths were in children under the age of 12. Efforts to combat malaria-carrying mosquitoes rely on fogging the home with chemical pesticides and sleeping under pesticide-soaked nets, and while this has reduced the occurrence of malaria in recent years, the result is nowhere near as effective as eradicating the Anopheles gambiae mosquito that carries the disease.
Pesticides, a known carcinogen for animals and humans, are a blunt instrument, says Anthony Shelton, a biologist and entomologist at Cornell University. "There are no pesticides so specific that they just get the animal you want to target. They get pollinators. They get predators and parasites. They negatively affect the ecosystem, and they get into our bodies." And it's not uncommon for insects to develop resistance to pesticides, necessitating the continuous development of new, more powerful chemicals to control them.
"The harm of insecticides is not debatable," says Shelton. With gene drives, the potential harm is less clear.
Shelton also points out that although genetic modification sounds radical, people have been altering the genes of animals since before recorded history, through the selective breeding of farm and domesticated animals. While critics of genetic modification decry the possibility of changing the trajectory of evolution in animals, "We've been doing it for centuries," says Shelton. "Gene drives are just a much faster way to do what we've been doing all along."
Still, one might argue that farms are closed experiments, because animals enclosed within farms don't mate with wild animals. This limits the impact of human changes on the larger ecosystem. And getting new genes to work their way through multiple generations in longer-lived animals through breeding can take centuries, which imposes the element of time to ascertain the relative benefits of any introduced change. Gene drives fast-forward change in ways that have never been harnessed before.
The unique thing about gene drives, Shelton says, is that they only affect the targeted species, because those animals will only breed with their own species. Although the Oxitec mosquitoes are modified but not imbued with a gene drive, they illustrate the point. Aedes aegypti will only mate with its own species, and not with any of the other 3,000 varieties of mosquito. According to Shelton, "If they were to disappear, it would have no effect on the fish, bats and birds that feed on them." But should gene drives become widely used, this won't always be true of animals that play a larger part in the food chain. This will be especially true if gene drives are used in mammals.
One factor, cited by both proponents of gene drives and those who want a complete moratorium on them, is that once a gene drive is released into the wild, animals tend to evolve strategies to resist them. In a 2017 article in Nature, Philip Messer, a population geneticist at Cornell, says that gene drives create "the ideal conditions for resistant organisms to flourish."
Sometimes, when CRISPR is used and the Cas9 enzyme cuts an allele soon after egg fertilization, the animal's repair mechanism, rather than creating a straight copy of the desired allele, inserts random DNA letters. The gene drive won't recognize the new sequence, and the change will slip through. In this way, nature has a way of overriding gene drives.
In caged experiments using CRISPR-modified mosquitoes, while the gene drive initially worked, resistance has developed fairly rapidly. Scientists working for Target Malaria, the massive anti-malaria enterprise funded by the Bill and Melinda Gates Foundation, are now working on developing a new version of a gene drive that is not so vulnerable to genetic resistance. But cage conditions are not representative of complex natural ecosystems, and to figure out how a modified species is going to affect the big picture, ultimately they will have to be tested in the wild.
Because there are so many unknowns, such testing is just too dangerous to undertake, according to environmentalists such as Dana Perls of the Friends of the Earth, an international consortium of environmental organizations headquartered in Amsterdam. "There's no safe way to experiment in the wild," she says. "Extinction is permanent, and to drive any species to extinction could have major environmental problems. At a time when we're seeing species disappearing at a high rate, we need to focus on safe processes and a slow approach rather than assume there's a silver bullet."
She cites a number of possible harmful outcomes from genetic modification, including the possible creation of dangerous hybrids that could be more effective at spreading disease and more resistant to pesticides. She points to a 2019 paper in Scientific Reports in which Yale researchers suggested there's evidence that genetically modified species can interbreed with organisms outside their own species. The researchers claimed that when Oxitec tested its modified Aedes aegypti mosquitoes in Brazil, the release resulted in a dangerous hybrid due to the altered animals breeding with two other varieties of mosquito. They suggested that the hybrid mosquito was more robust than the original gene drive mosquitoes.
The paper contributed to breathless headlines in the media and made a big splash with the anti-GMO community. However, it turned out that when other scientists reviewed the data, they found it didn't support the authors' claims. In a short time, the editors of Nature ran an Editorial Expression of Concern for the article, noting that of the insects examined by the researchers, none of them contained the transgenes of the released mosquitoes. Among multiple concerns, Nature found that the researchers didn't follow the released population for more than a short time, and that previous work from the same authors had shown that after a short time, transgenes would have faded from the population.
Of course, unintended consequences are always a concern any time we interfere with nature, says Michael Montague, a senior scholar at Johns Hopkins University's Center for Health Security. "Unpredictability is part of living in the world," he says. Still, he's relatively comfortable with the limited Florida Keys release.
"Even if one type of mosquito was eliminated in the Keys, the ecosystem wouldn't notice," he says. This is because of the thousands of other species of mosquito. He says that while the Keys initiative is ultimately a test, "Oxitec has done their due diligence."
Montague addressed another concern voiced by Perls. The Oxitec mosquitoes were developed so that the female larvae will only hatch in water containing the antibiotic tetracycline. Perls and others caution that, because of the widespread use of antibiotics, the drug inevitably makes its way into the water system, and could be present in the standing pools of water that mosquitoes mate and lay their eggs in.
It's highly unlikely that tetracycline would exist in concentrations high enough to make any difference, says Montague. "But even if it did happen, and the modified females hatched out and mated with wild males, many of their offspring would inherit the modification and only be able to hatch in tetracycline-laced water. The worst-case scenario would be that the pest control didn't work. Net effect: Zero," he says.
As for comparing GMO mosquitoes with insecticides, Montague says, "We 100 percent know insecticides have a harmful effect on human health, whereas modified [male] mosquitoes don't bite humans. They're essentially a chemical-free insecticide, and if there were to be some harmful effect on human health, it would have to be some complicated, convoluted effect" that no one has predicted.
It's not clear, though, given the transitory nature of self-limiting genetically modified insects, whether any effects on the ecosystem would be long-lasting. Certainly in the case of the Oxitec mosquitoes, any effect on the environment would likely be subtle. However, there are other species that are far more important to the food chain, and humans have been greatly impacting them for centuries, sometimes with disastrous effects.
The world's oceans are particularly vulnerable to the effects of human actions. "Codfish used to dominate the North Atlantic ecosystem," says Montague, but due to overfishing, there were huge changes to that ecosystem, including the expansion of their prey—lobsters, crabs and shrimp. The whole system got out of balance." The fish illustrate the international nature of the issues related to gene drives, because wild species have few boundaries and a change in one region can easily spread far and wide.
On the other hand, gene drives can be used for beneficial purposes beyond eliminating disease-carrying species. They could also be used to combat invasive species, fight crop-destroying insects, promote biodiversity, and give a leg up to endangered species that would otherwise die out.
Today nearly 90 percent of the world's islands have been invaded by disease-carrying rodents that have over-multiplied and are driving other island species to extinction. Common rodents such as rats and mice normally encounter a large number of predators in mainland territories, and this controls their numbers. Once they are introduced into island ecosystems, however, they have few predators and often become invasive. Because of this, they are a prevalent cause of the extinction of both animals and plants globally. The primary way to combat them has been to spread powerful toxicants that, when ingested, cause death. Not only has this inhumane practice had limited impact, the toxicants can be eaten by untargeted species and are toxic to humans.
The Genetic Biocontrol of Invasive Rodents program (GBIRd), an international consortium of scientists, ethicists, regulatory experts, sociologists, conservationists and others, is exploring the possible development of a genetically modified mouse that could be introduced to islands where rodents are invasive. Similar to the Oxitec mosquitoes, the mice would carry a modification that results in the appearance of only one sex, and they would also carry a gene drive. Theoretically, once they mate with the wild mice, all of the surviving offspring would be either male or female, and the species would disappear from the islands, giving other, threatened species an opportunity to revive.
GBIRd is moving slowly by design and is currently focused on asking if a genetically engineered mouse should be developed. The program is a potential model for how gene drives can be ethically developed with maximum foresight and the least impact on complex ecosystems. By first releasing a genetically engineered mouse on an island — likely years from now — the impact would naturally be contained within a limited locale.
Regulating GM Insects
While multiple agencies in the U.S. were involved in approving the release of the Oxitec mosquitoes, most experts agree that there is not a straightforward path to regulating genetically modified organisms released into the environment. Clearly, international regulation is needed as genetically modified organisms are released into open environments like the air and the ocean.
The United Nations' Convention on Biological Diversity, which oversees environmental issues at an international level, recently met to continue a process of hammering out voluntary protocols concerning gene drives. Multiple nations have already signed on to already-established protocols, but the United States has not and, according to Montague, is not expected to. "The U.S. will never be signatory to CBD agreements because agricultural companies are huge businesses" that may not see them as in their best interests, he says. Bans or limitations on the release of genetically modified organisms could limit crop yields, for example, thereby limiting profits.
Even if every nation signed on to international regulations of gene drives, cooperation is voluntary. The regulations wouldn't prevent bad actors from using the technology in nefarious ways, such as developing gene drives that can be used as weapons, according to Perls. An example would be unleashing a genetically modified invasive insect to destroy the crops of enemy nations. Or the releasing of a swarm of disease-carrying insects. But in this scenario, it would be very hard to limit the genetically modified species to a specific environment, and the bad actors could be unleashing disaster on themselves.
Because of the risks of misuse, scientists disagree on whether to openly share their gene drive research with others. But Montague believes that there should be a universal registry of gene drives, because "one gene drive can mess up another one. Two groups using the same species should know about each other," he says.
Ultimately, the decision of whether and when to release gene drives into nature rests with not one group, but with society as a whole. This includes not only diverse experts and regulatory bodies, but the general public, a group Oxitec spent considerable time and resources interacting with for their Florida Keys project. In the end, they gained approval for the initiative by a majority of Keys residents, but never gained a total consensus.
There's no escaping the fact that the use of gene drives is a nascent field, and even geneticists and regulators are still grapping with the best ways to develop, oversee, regulate, and control them. Much more data is needed to fully ascertain its risks and benefits.
Experts agree that the Oxitec venture isn't likely to have a noticeable effect on the larger ecosystem unless something truly catastrophic goes wrong. But following the GMO mosquitoes over time will give scientists more real-world data about the long-term effects of genetically altered species. If the release doesn't work, nothing about the ecosystem will change and Aedes aegypti will continue to be a menace to human health. But if something goes horribly wrong, it could hinder the field for years, if not forever.
On the other hand, if the Oxitec mosquitoes and other early initiatives achieve their goals of reducing disease, increasing crop yields, and protecting biodiversity, in the words of Anthony Shelton, "Maybe, 25 to 50 years from now, people will wonder what all the fuss was about."
Correction: The original version of this article mistakenly stated that the modified Oxitec mosquitoes would not be able to form a proper proboscis to bite humans. That is true for some modified mosquitoes but not the Oxitec ones, whose female offspring die off before they reach maturity. Additionally, the Oxitec release was not approved by the FDA and CDC, as originally stated. The FDA and CDC withdrew their role and passed the oversight to other regulatory entities.
Medical Breakthroughs Set to be Fast-Tracked by Innovative New Health Agency
Pippy Rogers, second from left, with her four siblings, who worry that they are at risk for Alzheimer's and are calling for an acceleration of research.
In 2007, Matthew Might's son, Bertrand, was born with a life-threatening disease that was so rare, doctors couldn't diagnose it. Might, a computer scientist and biologist, eventually realized, "Oh my gosh, he's the only patient in the world with this disease right now." To find effective treatments, new methodologies would need to be developed. But there was no process or playbook for doing that.
Might took it upon himself, along with a team of specialists, to try to find a cure. "What Bertrand really taught me was the visceral sense of urgency when there's suffering, and how to act on that," he said.
He calls it "the agency of urgency"—and patients with more common diseases, such as cancer and Alzheimer's, often feel that same need to take matters into their own hands, as they find their hopes for new treatments running up against bureaucratic systems designed to advance in small, steady steps, not leaps and bounds. "We all hope for a cure," said Florence "Pippy" Rogers, a 65-year-old volunteer with Georgia's chapter of the Alzheimer's Association. She lost her mother to the disease and, these days, worries about herself and her four siblings. "We need to keep accelerating research."
We have a fresh example of what can be achieved by fast-tracking discoveries in healthcare: Covid-19 vaccines.
President Biden has pushed for cancer moonshots since the disease took the life of his son, Beau, in 2015. His administration has now requested $6.5 billion to start a new agency in 2022, called the Advanced Research Projects Agency for Health, or ARPA-H, within the National Institutes of Health. It's based on DARPA, the Department of Defense agency known for hatching world-changing technologies such as drones, GPS and ARPANET, which became the internet.
We have a fresh example of what can be achieved by fast-tracking discoveries in healthcare: Covid-19 vaccines. "Operation Warp Speed was using ARPA-like principles," said Might. "It showed that in a moment of crisis, institutions like NIH can think in an ARPA-like way. So now the question is, why don't we do that all the time?"
But applying the DARPA model to health involves several challenging decisions. I asked experts what could be the hardest question facing advocates of ARPA-H: which health problems it should seek to address. "All the wonderful choices lead to the problem of which ones to choose and prioritize," said Sudip Parikh, CEO of the American Association for the Advancement of Science and executive publisher of the Science family of journals. "There is no objectively right answer."
The Agency of Urgency
ARPA-H will borrow at least three critical ingredients from DARPA: goal-oriented project managers, many from industry; aggressive public-private partnerships; and collaboration among fields that don't always interact. The DARPA concept has been applied to other purposes, including energy and homeland security, with promising results. "We're learning that 'ARPA-ism' is a franchisable model," said Might, a former principal investigator on DARPA projects.
The federal government already pours billions of dollars into advancing research on life-threatening diseases, with much of it channeled through the National Institutes of Health. But the purpose of ARPA-H "isn't just the usual suspects that NIH would fund," said David Walt, a Harvard biochemist, an innovator in gene sequencing and former chair of DARPA's Defense Science Research Council. Whereas some NIH-funded studies aim to gradually improve our understanding of diseases, ARPA-H projects will give full focus to real-world applications; they'll use essential findings from NIH research as starting points, drawing from them to rapidly engineer new technologies that could save lives.
And, ultimately, billions in healthcare costs, if ARPA-H lives up to its predecessor's track record; DARPA's breakthroughs have been economic game-changers, while its fail-fast approach—quickly pulling the plug on projects that aren't panning out—helps to avoid sunken costs. ARPA-H could fuel activities similar to the human genome project, which used existing research to map the base pairs that make up DNA, opening new doors for the biotech industry, sparking economic growth and creating hundreds of thousands of new jobs.
Despite a nearly $4 trillion health economy, "we aren't innovating when it comes to technological capabilities for health," said Liz Feld, president of the Suzanne Wright Foundation for pancreatic cancer.
Individual Diseases Ripe for Innovation
Although the need for innovation is clear, which diseases ARPA-H should tackle is less apparent. One important consideration when choosing health priorities could be "how many people suffer from a disease," said Nancy Kass, a professor of bioethics and public health at Johns Hopkins.
That perspective could justify cancer as a top objective. Cancer and heart disease have long been the two major killers in the U.S. Leonidas Platanias, professor of oncology at Northwestern and director of its cancer center, noted that we've already made significant progress on heart disease. "Anti-cholesterol drugs really have a wide impact," he said. "I don't want to compare one disease to another, but I think cancer may be the most challenging. We need even bigger breakthroughs." He wondered whether ARPA-H should be linked to the part of NIH dedicated to cancer, the National Cancer Institute, "to take maximum advantage of what happens" there.
Previous cancer moonshots have laid a foundation for success. And this sort of disease-by-disease approach makes sense in a way. "We know that concentrating on some diseases has led to treatments," said Parikh. "Think of spinal muscular atrophy or cystic fibrosis. Now, imagine if immune therapies were discovered ten years earlier."
But many advocates think ARPA-H should choose projects that don't revolve around any one disease. "It absolutely has to be disease agnostic," said Feld, president of the pancreatic cancer foundation. "We cannot reach ARPA-H's potential if it's subject to the advocacy of individual patient groups who think their disease is worse than the guy's disease next to them. That's not the way the DARPA model works." Platanias agreed that ARPA-H should "pick the highest concepts and developments that have the best chance" of success.
Finding Connections Between Diseases
Kass, the Hopkins bioethicist, believes that ARPA-H should walk a balance, with some projects focusing on specific diseases and others aspiring to solutions with broader applications, spanning multiple diseases. Being impartial, some have noted, might involve looking at the total "life years" saved by a health innovation; the more diseases addressed by a given breakthrough, the more years of healthy living it may confer. The social and economic value should increase as well.
For multiple payoffs, ARPA-H could concentrate on rare diseases, which can yield important insights for many other diseases, said Might. Every case of cancer and Alzheimer's is, in a way, its own rare disease. Cancer is a genetic disease, like his son Bertrand's rare disorder, and mutations vary widely across cancer patients. "It's safe to say that no two people have ever actually had the same cancer," said Might. In theory, solutions for rare diseases could help us understand how to individualize treatments for more common diseases.
Many experts I talked with support another priority for ARPA-H with implications for multiple diseases: therapies that slow down the aging process. "Aging is the greatest risk factor for every major disease that NIH is studying," said Matt Kaeberlein, a bio-gerontologist at the University of Washington. Yet, "half of one percent of the NIH budget goes to researching the biology of aging. An ARPA-H sized budget would push the field forward at a pace that's hard to imagine."
Might agreed. "It could take ARPA-H to get past the weird stigmas around aging-related research. It could have a tremendous impact on the field."
For example, ARPA-H could try to use mRNA technology to express proteins that affect biological aging, said Kaeberlein. It's an engineering project well-suited to the DARPA model. So is harnessing machine learning to identify biomarkers that assess how fast people are aging. Biological aging clocks, if validated, could quickly reveal whether proposed therapies for aging are working or not. "I think there's huge value in that," said Kaeberlein.
By delivering breakthroughs in computation, ARPA-H could improve diagnostics for many different diseases. That could include improving biowearables for continuously monitoring blood pressure—a hypothetical mentioned in the White House's concept paper on ARPA-H—and advanced imaging technologies. "The high cost of medical imaging is a leading reason why our healthcare costs are the highest in the world," said Feld. "There's no detection test for ALS. No brain detection for Alzheimer's. Innovations in detection technology would save on cost and human suffering."
Some biotech companies may be skeptical about the financial rewards of accelerating such technologies. But ARPA-H could fund public-private partnerships to "de-risk" biotech's involvement—an incentive that harkens back to the advance purchase contracts that companies got during Covid. (Some groups have suggested that ARPA-H could provide advance purchase agreements.)
Parikh is less bullish on creating diagnostics through ARPA-H. Like DARPA, Biden's health agency will enjoy some independence from federal oversight; it may even be located hundreds of miles from DC. That freedom affords some breathing room for innovation, but it could also make it tougher to ensure that algorithms fully consider diverse populations. "That part I really would like the government more involved in," Parikh said.
Might thinks ARPA-H should also explore innovations in clinical trials, which many patients and medical communities view as grindingly slow and requiring too many participants. "We can approve drugs for very tiny patient populations, even at the level of the individual," he said, while emphasizing the need for safety. But Platanias thinks the FDA has become much more flexible in recent years. In the cancer field, at least, "You now see faster approvals for more drugs. Having [more] shortcuts on clinical trial approvals is not necessarily a good idea."
With so many options on the table, ARPA-H needs to show the public a clear framework for measuring the value of potential projects. Kass warned that well-resourced advocates could skew the agency's priorities. They've affected health outcomes before, she noted; fundraising may partly explain larger increases in life expectancy for cystic fibrosis than sickle cell anemia. Engaging diverse communities is a must for ARPA-H. So are partnerships to get the agency's outputs to people who need them. "Research is half the equation," said Kass. "If we don't ensure implementation and access, who cares." The White House concept paper on ARPA-H made a similar point.
As Congress works on authorizing ARPA-H this year, Might is doing what he can to ensure better access to innovation on a patient-by-patient basis. Last year, his son, Bertrand, passed away suddenly from his disorder. He was 12. But Might's sense of urgency has persisted, as he directs the Precision Medicine Institute at the University of Alabama-Birmingham. That urgency "can be carried into an agency like ARPA-H," he said. "It guides what I do as I apply for funding, because I'm trying to build the infrastructure that other parents need. So they don't have to build it from scratch like I did."


