The U.S. must fund more biotech innovation – or other countries will catch up faster than you think

In the coming years, U.S. market share in biotech will decline unless the federal government makes investments to improve the quality and quantity of U.S. research, writes the author.
The U.S. has approximately 58 percent of the market share in the biotech sector, followed by China with 11 percent. However, this market share is the result of several years of previous research and development (R&D) – it is a present picture of what happened in the past. In the future, this market share will decline unless the federal government makes investments to improve the quality and quantity of U.S. research in biotech.
The effectiveness of current R&D can be evaluated in a variety of ways such as monies invested and the number of patents filed. According to the UNESCO Institute for Statistics, the U.S. spends approximately 2.7 percent of GDP on R&D ($476,459.0M), whereas China spends 2 percent ($346,266.3M). However, investment levels do not necessarily translate into goods that end up contributing to innovation.
Patents are a better indication of innovation. The biotech industry relies on patents to protect their investments, making patenting a key tool in the process of translating scientific discoveries that can ultimately benefit patients. In 2020, China filed 1,497,159 patents, a 6.9 percent increase in growth rate. In contrast, the U.S. filed 597,172, a 3.9 percent decline. When it comes to patents filed, China has approximately 45 percent of the world share compared to 18 percent for the U.S.
So how did we get here? The nature of science in academia allows scientists to specialize by dedicating several years to advance discovery research and develop new inventions that can then be licensed by biotech companies. This makes academic science critical to innovation in the U.S. and abroad.
Academic scientists rely on government and foundation grants to pay for R&D, which includes salaries for faculty, investigators and trainees, as well as monies for infrastructure, support personnel and research supplies. Of particular interest to academic scientists to cover these costs is government support such as Research Project Grants, also known as R01 grants, the oldest grant mechanism from the National Institutes of Health. Unfortunately, this funding mechanism is extremely competitive, as applications have a success rate of only about 20 percent. To maximize the chances of getting funded, investigators tend to limit the innovation of their applications, since a project that seems overambitious is discouraged by grant reviewers.
Considering the difficulty in obtaining funding, the limited number of opportunities for scientists to become independent investigators capable of leading their own scientific projects, and the salaries available to pay for scientists with a doctoral degree, it is not surprising that the U.S. is progressively losing its workforce for innovation.
This approach affects the future success of the R&D enterprise in the U.S. Pursuing less innovative work tends to produce scientific results that are more obvious than groundbreaking, and when a discovery is obvious, it cannot be patented, resulting in fewer inventions that go on to benefit patients. Even though there are governmental funding options available for scientists in academia focused on more groundbreaking and translational projects, those options are less coveted by academic scientists who are trying to obtain tenure and long-term funding to cover salaries and other associated laboratory expenses. Therefore, since only a small percent of projects gets funded, the likelihood of scientists interested in pursuing academic science or even research in general keeps declining over time.
Efforts to raise the number of individuals who pursue a scientific education are paying off. However, the number of job openings for those trainees to carry out independent scientific research once they graduate has proved harder to increase. These limitations are not just in the number of faculty openings to pursue academic science, which are in part related to grant funding, but also the low salary available to pay those scientists after they obtain their doctoral degree, which ranges from $53,000 to $65,000, depending on years of experience.
Thus, considering the difficulty in obtaining funding, the limited number of opportunities for scientists to become independent investigators capable of leading their own scientific projects, and the salaries available to pay for scientists with a doctoral degree, it is not surprising that the U.S. is progressively losing its workforce for innovation, which results in fewer patents filed.
Perhaps instead of encouraging scientists to propose less innovative projects in order to increase their chances of getting grants, the U.S. government should give serious consideration to funding investigators for their potential for success -- or the success they have already achieved in contributing to the advancement of science. Such a funding approach should be tiered depending on career stage or years of experience, considering that 42 years old is the median age at which the first R01 is obtained. This suggests that after finishing their training, scientists spend 10 years before they establish themselves as independent academic investigators capable of having the appropriate funds to train the next generation of scientists who will help the U.S. maintain or even expand its market share in the biotech industry for years to come. Patenting should be given more weight as part of the academic endeavor for promotion purposes, or governmental investment in research funding should be increased to support more than just 20 percent of projects.
Remaining at the forefront of biotech innovation will give us the opportunity to not just generate more jobs, but it will also allow us to attract the brightest scientists from all over the world. This talented workforce will go on to train future U.S. scientists and will improve our standard of living by giving us the opportunity to produce the next generation of therapies intended to improve human health.
This problem cannot rely on just one solution, but what is certain is that unless there are more creative changes in funding approaches for scientists in academia, eventually we may be saying “remember when the U.S. was at the forefront of biotech innovation?”
The Friday Five: How to exercise for cancer prevention
How to exercise for cancer prevention. Plus, a device that brings relief to back pain, ingredients for reducing Alzheimer's risk, the world's oldest disease could make you young again, and more.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- How to exercise for cancer prevention
- A device that brings relief to back pain
- Ingredients for reducing Alzheimer's risk
- Is the world's oldest disease the fountain of youth?
- Scared of crossing bridges? Your phone can help
New approach to brain health is sparking memories
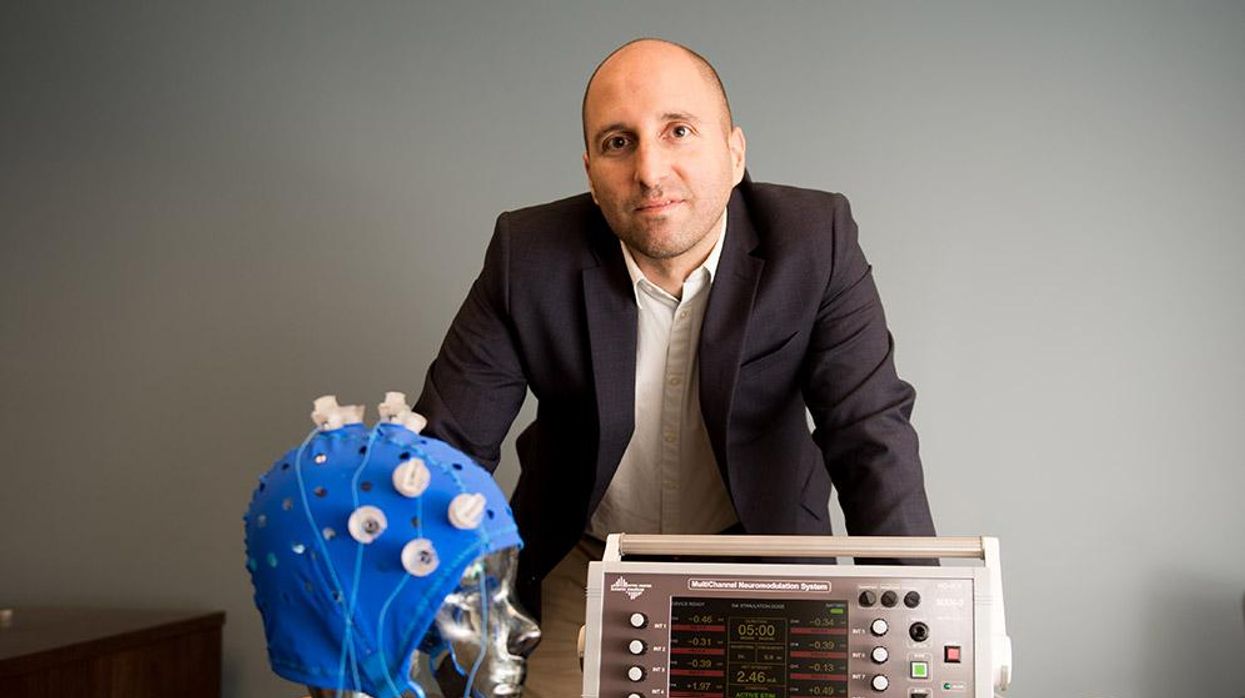
This fall, Robert Reinhart of Boston University published a study finding that electrical stimulation can boost memory - and Reinhart was surprised to discover the effects lasted a full month.
What if a few painless electrical zaps to your brain could help you recall names, perform better on Wordle or even ward off dementia?
This is where neuroscientists are going in efforts to stave off age-related memory loss as well as Alzheimer’s disease. Medications have shown limited effectiveness in reversing or managing loss of brain function so far. But new studies suggest that firing up an aging neural network with electrical or magnetic current might keep brains spry as we age.
Welcome to non-invasive brain stimulation (NIBS). No surgery or anesthesia is required. One day, a jolt in the morning with your own battery-operated kit could replace your wake-up coffee.
Scientists believe brain circuits tend to uncouple as we age. Since brain neurons communicate by exchanging electrical impulses with each other, the breakdown of these links and associations could be what causes the “senior moment”—when you can’t remember the name of the movie you just watched.
In 2019, Boston University researchers led by Robert Reinhart, director of the Cognitive and Clinical Neuroscience Laboratory, showed that memory loss in healthy older adults is likely caused by these disconnected brain networks. When Reinhart and his team stimulated two key areas of the brain with mild electrical current, they were able to bring the brains of older adult subjects back into sync — enough so that their ability to remember small differences between two images matched that of much younger subjects for at least 50 minutes after the testing stopped.
Reinhart wowed the neuroscience community once again this fall. His newer study in Nature Neuroscience presented 150 healthy participants, ages 65 to 88, who were able to recall more words on a given list after 20 minutes of low-intensity electrical stimulation sessions over four consecutive days. This amounted to a 50 to 65 percent boost in their recall.
Even Reinhart was surprised to discover the enhanced performance of his subjects lasted a full month when they were tested again later. Those who benefited most were the participants who were the most forgetful at the start.

An older person participates in Robert Reinhart's research on brain stimulation.
Robert Reinhart
Reinhart’s subjects only suffered normal age-related memory deficits, but NIBS has great potential to help people with cognitive impairment and dementia, too, says Krista Lanctôt, the Bernick Chair of Geriatric Psychopharmacology at Sunnybrook Health Sciences Center in Toronto. Plus, “it is remarkably safe,” she says.
Lanctôt was the senior author on a meta-analysis of brain stimulation studies published last year on people with mild cognitive impairment or later stages of Alzheimer’s disease. The review concluded that magnetic stimulation to the brain significantly improved the research participants’ neuropsychiatric symptoms, such as apathy and depression. The stimulation also enhanced global cognition, which includes memory, attention, executive function and more.
This is the frontier of neuroscience.
The two main forms of NIBS – and many questions surrounding them
There are two types of NIBS. They differ based on whether electrical or magnetic stimulation is used to create the electric field, the type of device that delivers the electrical current and the strength of the current.
Transcranial Current Brain Stimulation (tES) is an umbrella term for a group of techniques using low-wattage electrical currents to manipulate activity in the brain. The current is delivered to the scalp or forehead via electrodes attached to a nylon elastic cap or rubber headband.
Variations include how the current is delivered—in an alternating pattern or in a constant, direct mode, for instance. Tweaking frequency, potency or target brain area can produce different effects as well. Reinhart’s 2022 study demonstrated that low or high frequencies and alternating currents were uniquely tied to either short-term or long-term memory improvements.
Sessions may be 20 minutes per day over the course of several days or two weeks. “[The subject] may feel a tingling, warming, poking or itching sensation,” says Reinhart, which typically goes away within a minute.
The other main approach to NIBS is Transcranial Magnetic Simulation (TMS). It involves the use of an electromagnetic coil that is held or placed against the forehead or scalp to activate nerve cells in the brain through short pulses. The stimulation is stronger than tES but similar to a magnetic resonance imaging (MRI) scan.
The subject may feel a slight knocking or tapping on the head during a 20-to-60-minute session. Scalp discomfort and headaches are reported by some; in very rare cases, a seizure can occur.
No head-to-head trials have been conducted yet to evaluate the differences and effectiveness between electrical and magnetic current stimulation, notes Lanctôt, who is also a professor of psychiatry and pharmacology at the University of Toronto. Although TMS was approved by the FDA in 2008 to treat major depression, both techniques are considered experimental for the purpose of cognitive enhancement.
“One attractive feature of tES is that it’s inexpensive—one-fifth the price of magnetic stimulation,” Reinhart notes.
Don’t confuse either of these procedures with the horrors of electroconvulsive therapy (ECT) in the 1950s and ‘60s. ECT is a more powerful, riskier procedure used only as a last resort in treating severe mental illness today.
Clinical studies on NIBS remain scarce. Standardized parameters and measures for testing have not been developed. The high heterogeneity among the many existing small NIBS studies makes it difficult to draw general conclusions. Few of the studies have been replicated and inconsistencies abound.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Lanctôt’s meta-analysis showed improvements in global cognition from delivering the magnetic form of the stimulation to people with Alzheimer’s, and this finding was replicated inan analysis in the Journal of Prevention of Alzheimer’s Disease this fall. Neither meta-analysis found clear evidence that applying the electrical currents, was helpful for Alzheimer’s subjects, but Lanctôt suggests this might be merely because the sample size for tES was smaller compared to the groups that received TMS.
At the same time, London neuroscientist Marco Sandrini, senior lecturer in psychology at the University of Roehampton, critically reviewed a series of studies on the effects of tES on episodic memory. Often declining with age, episodic memory relates to recalling a person’s own experiences from the past. Sandrini’s review concluded that delivering tES to the prefrontal or temporoparietal cortices of the brain might enhance episodic memory in older adults with Alzheimer’s disease and amnesiac mild cognitive impairment (the predementia phase of Alzheimer’s when people start to have symptoms).
Researchers readily tick off studies needed to explore, clarify and validate existing NIBS data. What is the optimal stimulus session frequency, spacing and duration? How intense should the stimulus be and where should it be targeted for what effect? How might genetics or degree of brain impairment affect responsiveness? Would adjunct medication or cognitive training boost positive results? Could administering the stimulus while someone sleeps expedite memory consolidation?
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain.
While Sandrini’s review reported improvements induced by tES in the recall or recognition of words and images, there is no evidence it will translate into improvements in daily activities. This is another question that will require more research and testing, Sandrini notes.
Scientists are still lacking so much fundamental knowledge about the brain and how it works, says Reinhart. “We don’t know how information is represented in the brain or how it’s carried forward in time. It’s more complex than physics.”
Where the science is headed
Learning how to apply precision medicine to NIBS is the next focus in advancing this technology, says Shankar Tumati, a post-doctoral fellow working with Lanctôt.
There is great variability in each person’s brain anatomy—the thickness of the skull, the brain’s unique folds, the amount of cerebrospinal fluid. All of these structural differences impact how electrical or magnetic stimulation is distributed in the brain and ultimately the effects.
Using MRI or another brain scan along with computational modeling of the current flow, a clinician could create a treatment that is customized to each person’s brain, from where to put the electrodes to determining the exact dose and duration of stimulation needed to achieve lasting results, Sandrini says.
Above all, most neuroscientists say that largescale research studies over long periods of time are necessary to confirm the safety and durability of this therapy for the purpose of boosting memory. Short of that, there can be no FDA approval or medical regulation for this clinical use.
Lanctôt urges people to seek out clinical NIBS trials in their area if they want to see the science advance. “That is how we’ll find the answers,” she says, predicting it will be 5 to 10 years to develop each additional clinical application of NIBS. Ultimately, she predicts that reigning in Alzheimer’s disease and mild cognitive impairment will require a multi-pronged approach that includes lifestyle and medications, too.
Sandrini believes that scientific efforts should focus on preventing or delaying Alzheimer’s. “We need to start intervention earlier—as soon as people start to complain about forgetting things,” he says. “Changes in the brain start 10 years before [there is a problem]. Once Alzheimer’s develops, it is too late.”