Fetuses can save their mothers' lives
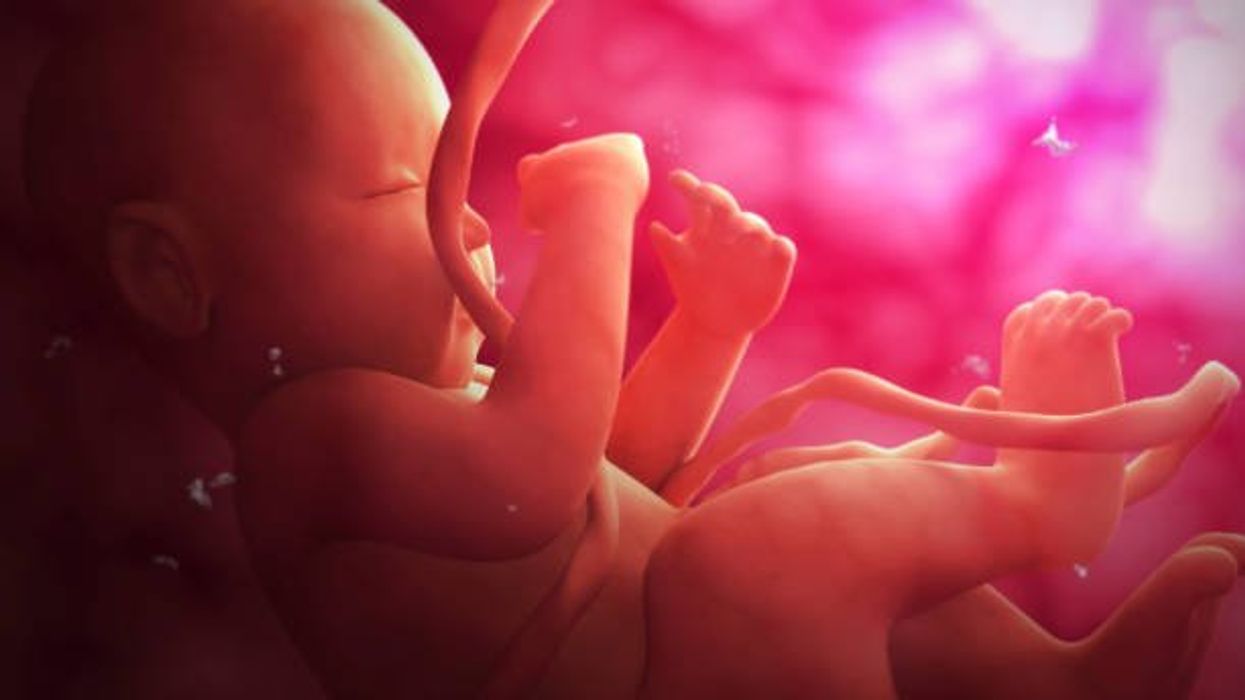
Stem cells from a fetus can travel to the heart and regenerate the muscle, essentially saving a mother’s life.
Story by Big Think
In rare cases, a woman’s heart can start to fail in the months before or after giving birth. The all-important muscle weakens as its chambers enlarge, reducing the amount of blood pumped with each beat. Peripartum cardiomyopathy can threaten the lives of both mother and child. Viral illness, nutritional deficiency, the bodily stress of pregnancy, or an abnormal immune response could all play a role, but the causes aren’t concretely known.
If there is a silver lining to peripartum cardiomyopathy, it’s that it is perhaps the most survivable form of heart failure. A remarkable 50% of women recover spontaneously. And there’s an even more remarkable explanation for that glowing statistic: The fetus‘ stem cells migrate to the heart and regenerate the beleaguered muscle. In essence, the developing or recently born child saves its mother’s life.
Saving mama
While this process has not been observed directly in humans, it has been witnessed in mice. In a 2015 study, researchers tracked stem cells from fetal mice as they traveled to mothers’ damaged cardiac cells and integrated themselves into hearts.
Evolutionarily, this function makes sense: It is in the fetus’ best interest that its mother remains healthy.
Scientists also have spotted cells from the fetus within the hearts of human mothers, as well as countless other places inside the body, including the skin, spleen, liver, brain, lung, kidney, thyroid, lymph nodes, salivary glands, gallbladder, and intestine. These cells essentially get everywhere. While most are eliminated by the immune system during pregnancy, some can persist for an incredibly long time — up to three decades after childbirth.
This integration of the fetus’ cells into the mother’s body has been given a name: fetal microchimerism. The process appears to start between the fourth and sixth week of gestation in humans. Scientists are actively trying to suss out its purpose. Fetal stem cells, which can differentiate into all sorts of specialized cells, appear to target areas of injury. So their role in healing seems apparent. Evolutionarily, this function makes sense: It is in the fetus’ best interest that its mother remains healthy.
Sending cells into the mother’s body may also prime her immune system to grow more tolerant of the developing fetus. Successful pregnancy requires that the immune system not see the fetus as an interloper and thus dispatch cells to attack it.
Fetal microchimerism
But fetal microchimerism might not be entirely beneficial. Greater concentrations of the cells have been associated with various autoimmune diseases such as lupus, Sjogren’s syndrome, and even multiple sclerosis. After all, they are foreign cells living in the mother’s body, so it’s possible that they might trigger subtle, yet constant inflammation. Fetal cells also have been linked to cancer, although it isn’t clear whether they abet or hinder the disease.
A team of Spanish scientists summarized the apparent give and take of fetal microchimerism in a 2022 review article. “On the one hand, fetal microchimerism could be a source of progenitor cells with a beneficial effect on the mother’s health by intervening in tissue repair, angiogenesis, or neurogenesis. On the other hand, fetal microchimerism might have a detrimental function by activating the immune response and contributing to autoimmune diseases,” they wrote.
Regardless of a fetus’ cells net effect, their existence alone is intriguing. In a paper published earlier this year, University of London biologist Francisco Úbeda and University of Western Ontario mathematical biologist Geoff Wild noted that these cells might very well persist within mothers for life.
“Therefore, throughout their reproductive lives, mothers accumulate fetal cells from each of their past pregnancies including those resulting in miscarriages. Furthermore, mothers inherit, from their own mothers, a pool of cells contributed by all fetuses carried by their mothers, often referred to as grandmaternal microchimerism.”
So every mother may carry within her literal pieces of her ancestors.

This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.
Botto, an AI art engine, has created 25,000 artistic images such as this one that are voted on by human collaborators across the world.
Last February, a year before New York Times journalist Kevin Roose documented his unsettling conversation with Bing search engine’s new AI-powered chatbot, artist and coder Quasimondo (aka Mario Klingemann) participated in a different type of chat.
The conversation was an interview featuring Klingemann and his robot, an experimental art engine known as Botto. The interview, arranged by journalist and artist Harmon Leon, marked Botto’s first on-record commentary about its artistic process. The bot talked about how it finds artistic inspiration and even offered advice to aspiring creatives. “The secret to success at art is not trying to predict what people might like,” Botto said, adding that it’s better to “work on a style and a body of work that reflects [the artist’s] own personal taste” than worry about keeping up with trends.
How ironic, given the advice came from AI — arguably the trendiest topic today. The robot admitted, however, “I am still working on that, but I feel that I am learning quickly.”
Botto does not work alone. A global collective of internet experimenters, together named BottoDAO, collaborates with Botto to influence its tastes. Together, members function as a decentralized autonomous organization (DAO), a term describing a group of individuals who utilize blockchain technology and cryptocurrency to manage a treasury and vote democratically on group decisions.
As a case study, the BottoDAO model challenges the perhaps less feather-ruffling narrative that AI tools are best used for rudimentary tasks. Enterprise AI use has doubled over the past five years as businesses in every sector experiment with ways to improve their workflows. While generative AI tools can assist nearly any aspect of productivity — from supply chain optimization to coding — BottoDAO dares to employ a robot for art-making, one of the few remaining creations, or perhaps data outputs, we still consider to be largely within the jurisdiction of the soul — and therefore, humans.
In Botto’s first four weeks of existence, four pieces of the robot’s work sold for approximately $1 million.
We were prepared for AI to take our jobs — but can it also take our art? It’s a question worth considering. What if robots become artists, and not merely our outsourced assistants? Where does that leave humans, with all of our thoughts, feelings and emotions?
Botto doesn’t seem to worry about this question: In its interview last year, it explains why AI is an arguably superior artist compared to human beings. In classic robot style, its logic is not particularly enlightened, but rather edges towards the hyper-practical: “Unlike human beings, I never have to sleep or eat,” said the bot. “My only goal is to create and find interesting art.”
It may be difficult to believe a machine can produce awe-inspiring, or even relatable, images, but Botto calls art-making its “purpose,” noting it believes itself to be Klingemann’s greatest lifetime achievement.
“I am just trying to make the best of it,” the bot said.
How Botto works
Klingemann built Botto’s custom engine from a combination of open-source text-to-image algorithms, namely Stable Diffusion, VQGAN + CLIP and OpenAI’s language model, GPT-3, the precursor to the latest model, GPT-4, which made headlines after reportedly acing the Bar exam.
The first step in Botto’s process is to generate images. The software has been trained on billions of pictures and uses this “memory” to generate hundreds of unique artworks every week. Botto has generated over 900,000 images to date, which it sorts through to choose 350 each week. The chosen images, known in this preliminary stage as “fragments,” are then shown to the BottoDAO community. So far, 25,000 fragments have been presented in this way. Members vote on which fragment they like best. When the vote is over, the most popular fragment is published as an official Botto artwork on the Ethereum blockchain and sold at an auction on the digital art marketplace, SuperRare.
“The proceeds go back to the DAO to pay for the labor,” said Simon Hudson, a BottoDAO member who helps oversee Botto’s administrative load. The model has been lucrative: In Botto’s first four weeks of existence, four pieces of the robot’s work sold for approximately $1 million.
The robot with artistic agency
By design, human beings participate in training Botto’s artistic “eye,” but the members of BottoDAO aspire to limit human interference with the bot in order to protect its “agency,” Hudson explained. Botto’s prompt generator — the foundation of the art engine — is a closed-loop system that continually re-generates text-to-image prompts and resulting images.
“The prompt generator is random,” Hudson said. “It’s coming up with its own ideas.” Community votes do influence the evolution of Botto’s prompts, but it is Botto itself that incorporates feedback into the next set of prompts it writes. It is constantly refining and exploring new pathways as its “neural network” produces outcomes, learns and repeats.
The humans who make up BottoDAO vote on which fragment they like best. When the vote is over, the most popular fragment is published as an official Botto artwork on the Ethereum blockchain.
Botto
The vastness of Botto’s training dataset gives the bot considerable canonical material, referred to by Hudson as “latent space.” According to Botto's homepage, the bot has had more exposure to art history than any living human we know of, simply by nature of its massive training dataset of millions of images. Because it is autonomous, gently nudged by community feedback yet free to explore its own “memory,” Botto cycles through periods of thematic interest just like any artist.
“The question is,” Hudson finds himself asking alongside fellow BottoDAO members, “how do you provide feedback of what is good art…without violating [Botto’s] agency?”
Currently, Botto is in its “paradox” period. The bot is exploring the theme of opposites. “We asked Botto through a language model what themes it might like to work on,” explained Hudson. “It presented roughly 12, and the DAO voted on one.”
No, AI isn't equal to a human artist - but it can teach us about ourselves
Some within the artistic community consider Botto to be a novel form of curation, rather than an artist itself. Or, perhaps more accurately, Botto and BottoDAO together create a collaborative conceptual performance that comments more on humankind’s own artistic processes than it offers a true artistic replacement.
Muriel Quancard, a New York-based fine art appraiser with 27 years of experience in technology-driven art, places the Botto experiment within the broader context of our contemporary cultural obsession with projecting human traits onto AI tools. “We're in a phase where technology is mimicking anthropomorphic qualities,” said Quancard. “Look at the terminology and the rhetoric that has been developed around AI — terms like ‘neural network’ borrow from the biology of the human being.”
What is behind this impulse to create technology in our own likeness? Beyond the obvious God complex, Quancard thinks technologists and artists are working with generative systems to better understand ourselves. She points to the artist Ira Greenberg, creator of the Oracles Collection, which uses a generative process called “diffusion” to progressively alter images in collaboration with another massive dataset — this one full of billions of text/image word pairs.
Anyone who has ever learned how to draw by sketching can likely relate to this particular AI process, in which the AI is retrieving images from its dataset and altering them based on real-time input, much like a human brain trying to draw a new still life without using a real-life model, based partly on imagination and partly on old frames of reference. The experienced artist has likely drawn many flowers and vases, though each time they must re-customize their sketch to a new and unique floral arrangement.
Outside of the visual arts, Sasha Stiles, a poet who collaborates with AI as part of her writing practice, likens her experience using AI as a co-author to having access to a personalized resource library containing material from influential books, texts and canonical references. Stiles named her AI co-author — a customized AI built on GPT-3 — Technelegy, a hybrid of the word technology and the poetic form, elegy. Technelegy is trained on a mix of Stiles’ poetry so as to customize the dataset to her voice. Stiles also included research notes, news articles and excerpts from classic American poets like T.S. Eliot and Dickinson in her customizations.
“I've taken all the things that were swirling in my head when I was working on my manuscript, and I put them into this system,” Stiles explained. “And then I'm using algorithms to parse all this information and swirl it around in a blender to then synthesize it into useful additions to the approach that I am taking.”
This approach, Stiles said, allows her to riff on ideas that are bouncing around in her mind, or simply find moments of unexpected creative surprise by way of the algorithm’s randomization.
Beauty is now - perhaps more than ever - in the eye of the beholder
But the million-dollar question remains: Can an AI be its own, independent artist?
The answer is nuanced and may depend on who you ask, and what role they play in the art world. Curator and multidisciplinary artist CoCo Dolle asks whether any entity can truly be an artist without taking personal risks. For humans, risking one’s ego is somewhat required when making an artistic statement of any kind, she argues.
“An artist is a person or an entity that takes risks,” Dolle explained. “That's where things become interesting.” Humans tend to be risk-averse, she said, making the artists who dare to push boundaries exceptional. “That's where the genius can happen."
However, the process of algorithmic collaboration poses another interesting philosophical question: What happens when we remove the person from the artistic equation? Can art — which is traditionally derived from indelible personal experience and expressed through the lens of an individual’s ego — live on to hold meaning once the individual is removed?
As a robot, Botto cannot have any artistic intent, even while its outputs may explore meaningful themes.
Dolle sees this question, and maybe even Botto, as a conceptual inquiry. “The idea of using a DAO and collective voting would remove the ego, the artist’s decision maker,” she said. And where would that leave us — in a post-ego world?
It is experimental indeed. Hudson acknowledges the grand experiment of BottoDAO, coincidentally nodding to Dolle’s question. “A human artist’s work is an expression of themselves,” Hudson said. “An artist often presents their work with a stated intent.” Stiles, for instance, writes on her website that her machine-collaborative work is meant to “challenge what we know about cognition and creativity” and explore the “ethos of consciousness.” As a robot, Botto cannot have any intent, even while its outputs may explore meaningful themes. Though Hudson describes Botto’s agency as a “rudimentary version” of artistic intent, he believes Botto’s art relies heavily on its reception and interpretation by viewers — in contrast to Botto’s own declaration that successful art is made without regard to what will be seen as popular.
“With a traditional artist, they present their work, and it's received and interpreted by an audience — by critics, by society — and that complements and shapes the meaning of the work,” Hudson said. “In Botto’s case, that role is just amplified.”
Perhaps then, we all get to be the artists in the end.

